Catalyst for preparing carbon monoxide by electrochemically reducing carbon dioxide and preparation method and application thereof
A carbon dioxide, carbon monoxide technology, applied in physical/chemical process catalysts, chemical instruments and methods, organic compound/hydride/coordination complex catalysts, etc., can solve safety problems, complex catalyst synthesis steps, unfavorable large-scale macroscale Preparation and other problems, to achieve good solubility and dispersibility, to achieve large-scale industrial production and application, to solve the effects of poisoning and pollution problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
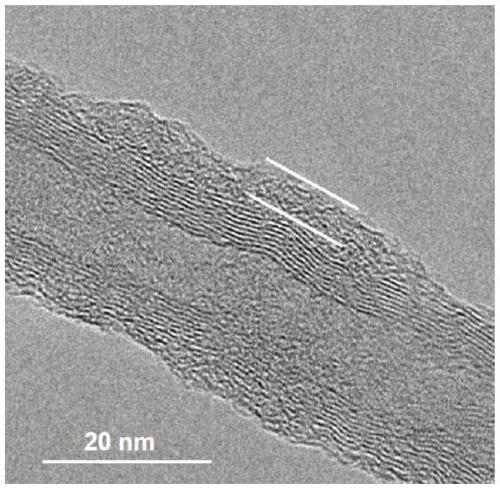
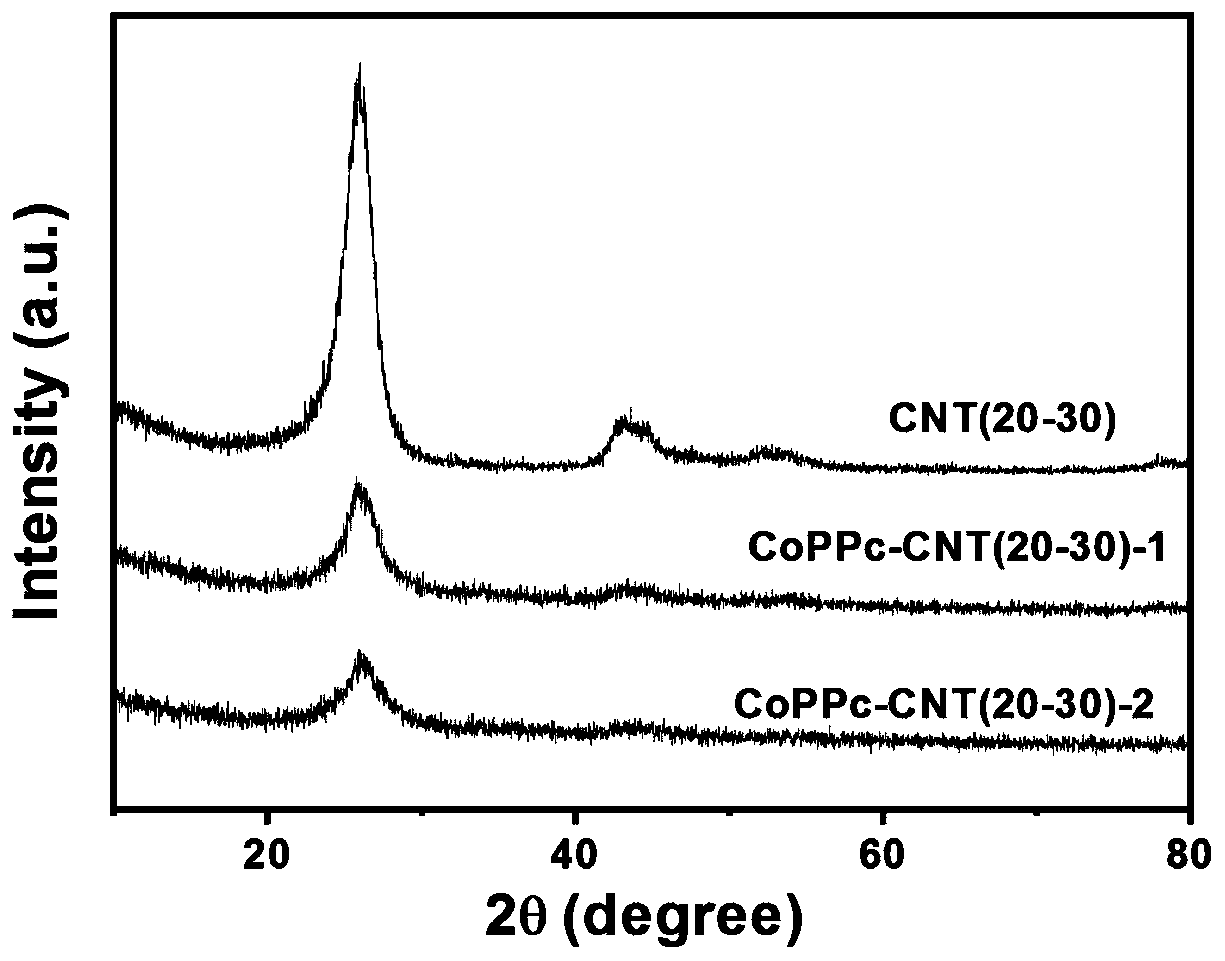
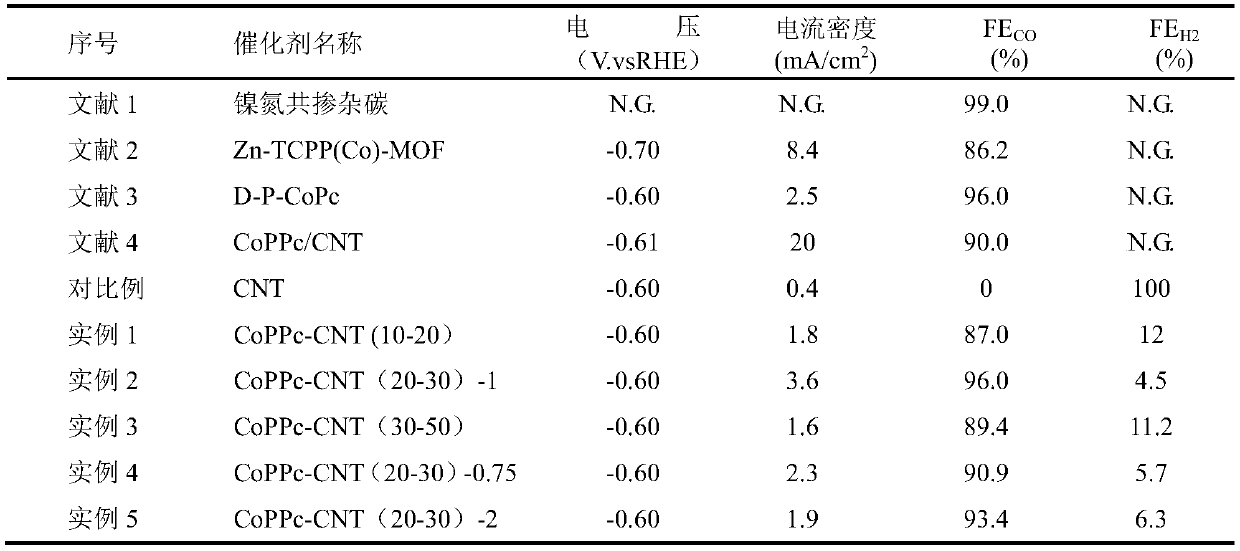
[0050] In this example, the preparation of CoPPc-CNT (10-20) catalyst is carried out, polymer layer: carbon tube mass: Co=1:1:0.16.
[0051] S1: Weigh 100 mg of carbon nanotubes with an outer diameter of 10-20 nm, place them in 80 ml of ethanol / water, and ultrasonically disperse them for 30 min to make them homogeneous. The power of the ultrasonic instrument is 53 kHz.
[0052] S2: Weigh 100mg of 2,4,5-benzenetetracarbonitrile and 86g of 1,8-diazabicyclo[5.4.0]undec-7-ene, weigh 27mg of anhydrous cobalt chloride, and mix the above Both were ultrasonically dispersed in ethanol / water containing carbon nanotubes.
[0053] S3: Transfer the dispersion containing the above precursor to a 100ml tetrafluoroethylene lining, place the lining in a stainless steel container, seal it and put it in an oven, and react at 180°C for 8h.
[0054] S4: After the reaction is completed, cool to room temperature, divide the mixed solution into centrifuge tubes, centrifuge at 8000 rpm, and then wash...
Embodiment 2
[0059] This example is the preparation of CoPPc-CNT(20-30)-1 catalyst, wherein polymer layer: carbon tube mass: Co=1:1:0.16.
[0060] S1: Weigh 100 mg of carbon nanotubes with an outer diameter of 20-30 nm, place them in 80 ml of ethanol / water, and ultrasonically disperse them for 30 min to make them homogeneous. The power of the ultrasonic instrument is 53 kHz.
[0061] S2: Weigh 100mg of 1,2,4,5-pyrene tetracarbonitrile and 86g of 1,8-diazabicyclo[5.4.0]undec-7-ene, and ultrasonically disperse 27mg of anhydrous cobalt chloride in the tube of ethanol / water.
[0062] S3: Transfer the dispersion containing the above precursor to a 100ml tetrafluoroethylene lining, place the lining in a stainless steel container, seal it and put it in an oven, and react at 180°C for 8h.
[0063] S4: After the reaction is completed, cool to room temperature, divide the mixed solution into centrifuge tubes, centrifuge at 8000 rpm, and wash twice with alcohol and water respectively. The amount of ...
Embodiment 3
[0070] This example is the preparation of CoPPc-CNT (30-50) catalyst, wherein polymer layer: carbon tube mass: Co = 1:1:0.16.
[0071] S1: Weigh 100 mg of carbon nanotubes with an outer diameter of 30-50 nm, place them in 80 ml of ethanol / water, and ultrasonically disperse them for 30 min to make them homogeneous. The power of the ultrasonic instrument is 53 kHz.
[0072] S2: Weigh 100mg of 1,2,4,5-pyrene tetracarbonitrile and 86g of 1,8-diazabicyclo[5.4.0]undec-7-ene, and ultrasonically disperse 27mg of anhydrous cobalt chloride in the tube of ethanol / water.
[0073] S3: Transfer the dispersion containing the above precursor to a 100ml tetrafluoroethylene lining, place the lining in a stainless steel container, seal it and put it in an oven, and react at 180°C for 8h.
[0074] S4: After the reaction is completed, cool to room temperature, divide the mixed solution into centrifuge tubes, centrifuge at 8000 rpm, and wash twice with alcohol and water respectively. The amount of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com