A histone acetyltransferase chrono-current sensor based on gold-palladium nanoflowers/graphene composite and its application
An acetyltransferase and current sensor technology, applied in the field of functional biological materials and biosensing, to achieve high-sensitivity detection, fast detection speed, and good selectivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1 Preparation of sensor
[0032](1) Acetyl antibody-gold palladium nanoflower / graphene composite material (Ab Ac - Preparation of AuPd@GO)
[0033] Disperse 0.5g graphene (GO) in 20mL deionized water and sonicate for 0.5h, then add 2.5mL 1g / L chloroauric acid (HAuCl 4 ) solution and 2.5mL 1g / L of chloropalladium acid (H 2 PdCl 4 ) solution, stirred evenly by magnetic force for 0.5h, placed under xenon arc lamp irradiation for 0.5h, then transferred to a drying oven, and reacted at 60°C for 24h. Finally, the sample was placed in a hydrogen tube furnace at 300 °C for 2 h. Disperse the prepared AuPd@GO in deionized water to obtain a 1 mg / mL AuPd@GO dispersion for later use.
[0034] Take 100 μL 1g / L acetyl antibody (Ab Ac ) placed in a He-Ne laser therapeutic apparatus for 30s irradiation, then added to 1mL of the above-mentioned AuPd@GO dispersion, and reacted at 37°C for 4h.
[0035] (2) Preparation of histone acetyltransferase timing-amperometric senso...
Embodiment 2
[0043] Example 2 Electrochemical response with or without p300
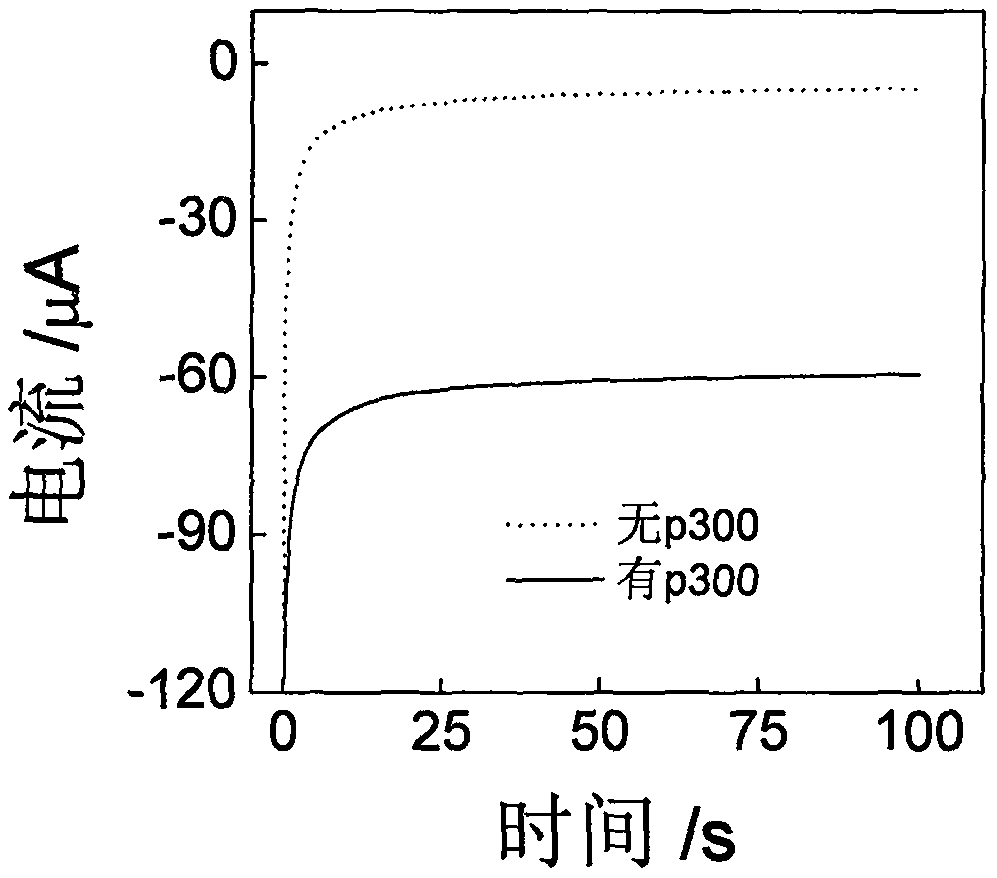
[0044] The histone acetyltransferase chrono-current sensor based on the gold-palladium nanoflower / graphene composite material and its application, using Example 1 to prepare our biosensor to explore the detection of histone acetyltransferase. See figure 2 , In the absence of p300, the sensor has basically no electrochemical response in PBS (0.1M, pH 7.0), while in the presence of p300, there is an obvious electrochemical response, which proves that the sensor can be used for p300 activity detection.
Embodiment 3
[0045] Example 3 Detection of p300 activity
[0046] Histone acetyltransferase timing-current sensor based on gold-palladium nanoflower / graphene composite material and its application, the preparation steps of the sensor are the same as in Example 1, during the acetylation reaction, the concentration of p300 and the concentration of p300 are changed successively For: 0, 0.001, 0.005, 0.01, 0.02, 0.05, 0.1, 0.2, 0.5, 1, 2, 5, 10, 20, 50, 100, 150, 200, 300, 500, 700, 1000 nM, subsequently used in the preparation sensor. Record the electrochemical response of the sensor in PBS (0.1M, pH 7.0). According to the experimental results, a series of electrochemical response curves corresponding to different concentrations of p300 are obtained, and the quantitative relationship between the electrochemical response current and the concentration of p300 is established. The quantitative relationship between the two determines the concentration of p300 in the sample to be tested. Experime...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com