Application of Juniper plant polysaccharide in preparing medicine for preventing and treating viral acute lung injury
A technology of acute lung injury and plant polysaccharides, applied in the preparation of drugs for the prevention and treatment of viral acute lung injury, new drug application field of plant polysaccharides of Cupressaceae Juniper
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Cedar take medicine 100g, pulverized, cold soak in 95% ethanol extracted three times, dried dregs home ventilation at room temperature and then extracted 3 times with hot water, filtered, and the combined extracts were concentrated and ethanol was added to a solution of alcohol an amount of 80%, the precipitate reconstituted in order to free protein trichloroacetic acid, centrifuged, the supernatant was dialyzed against water for 3 days, the dialysate was concentrated and freeze-dried to obtain the total polysaccharide. Sulfuric acid - phenol as measured cedar Polysaccharide content 81.2%; inter-hydroxybiphenyl as measured using the Cedar polysaccharides uronic acid content of 11.1%; protein using Coomassie brilliant blue as measured cedar polysaccharide content 1.1 %.
Embodiment 2
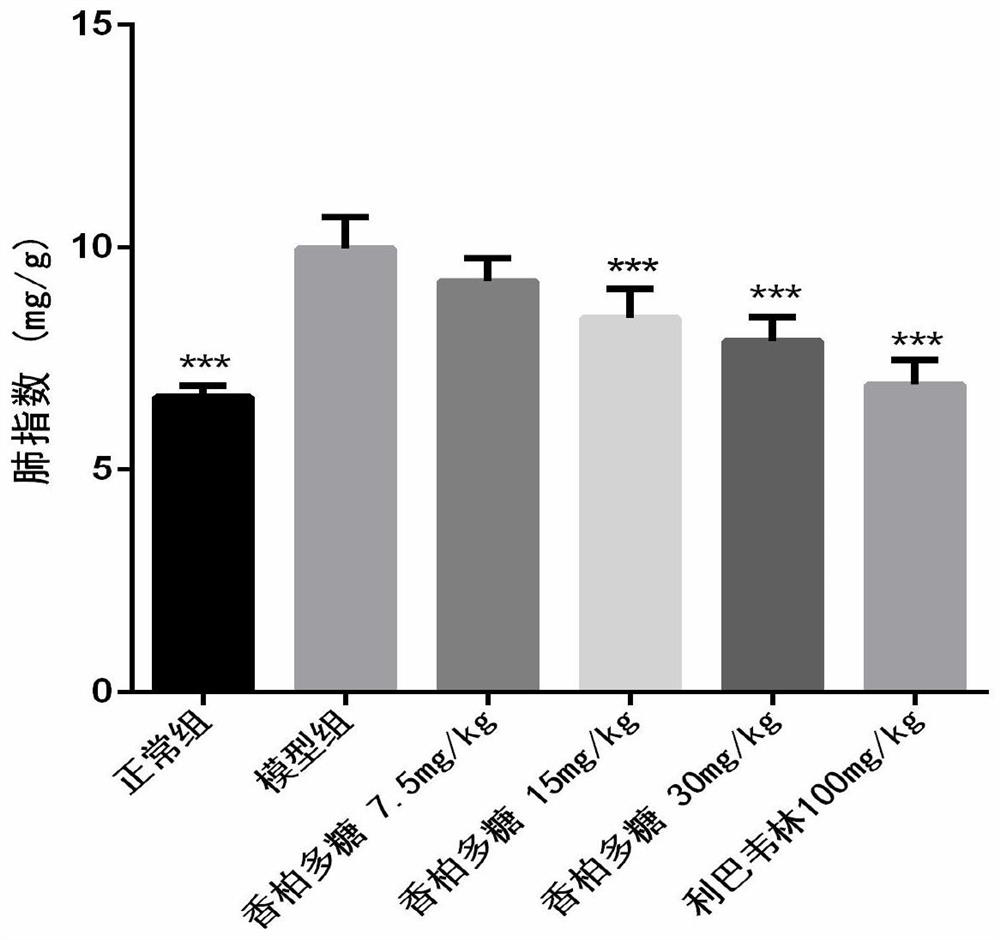
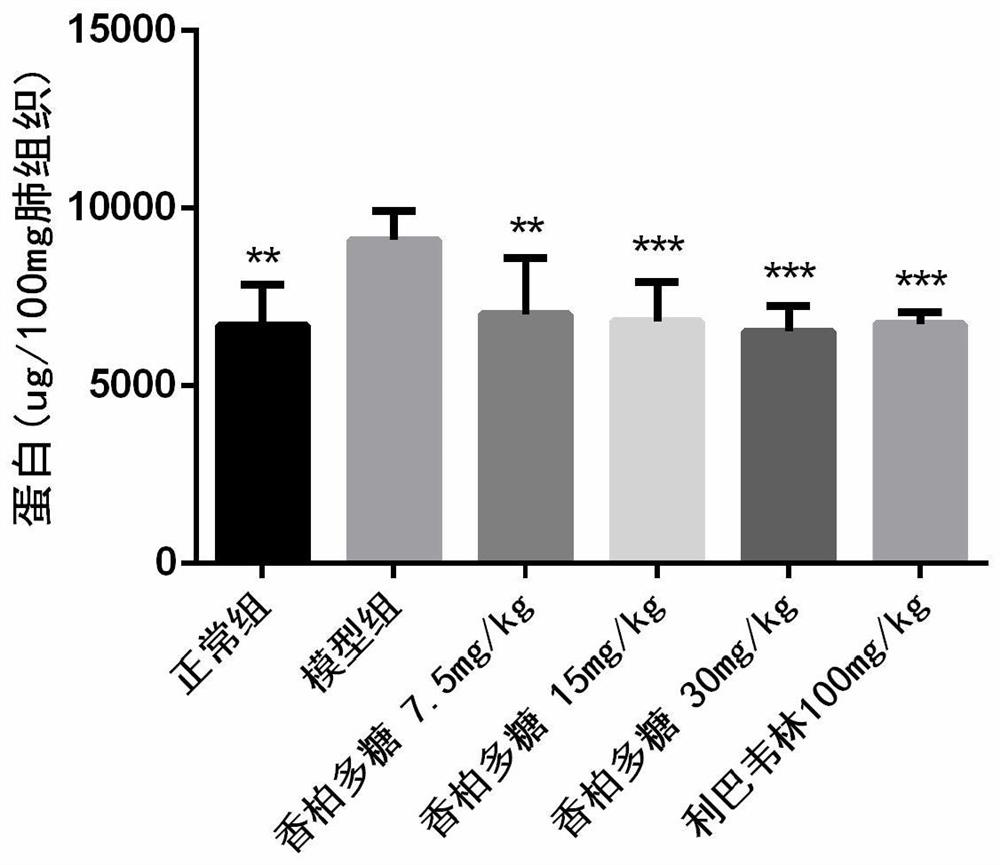
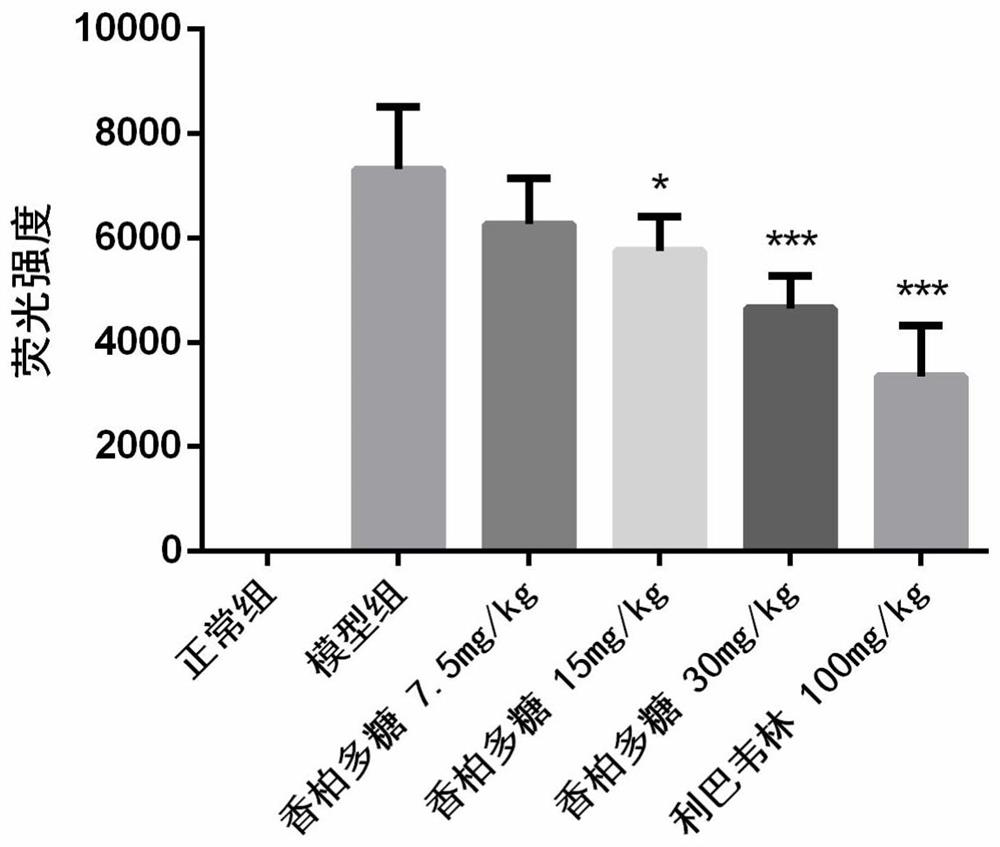
[0039] BALB / C 36 mice (14-16g), were randomly divided into 6 groups (N, M, A, B, C, P): N is the normal group, M is a group H1N1 virus model group, A is a group 7.5mg / kg, group B polysaccharide cedar cedar polysaccharide 15mg / kg, group C polysaccharide cedar 30mg / kg, P group, positive drug ribavirin 100mg / kg, n = 6, all animals prop tail vein injection of propofol anesthesia, N intranasally 1640 30μL group as a control; the other group intranasal infection 5LD 50 H1N1 virus solution 30 L, two hours after the oral administration H1N1 infection, N group and M group given 0.5% CMC oral administration, and as a normal viral control, administered once a day for consecutive four days, H1N1 viruses 96h after weighing the weight, eyeball blood carefully cut the whole lung, blotted dry blood filter paper, weighing records; carefully cut on the lobe in 10% formalin; middle right lung, the left lung and the lower lobe rinse with saline, was placed stored at -80 ℃. Lung tissue after...
Embodiment 3
[0046] BALB / C mice (14-16 g), which were randomly divided into 6 groups (N, M, A, B, C, P): N group as a normal control group, and the M group was H1N1 viral model group, Group A For the cedar polysaccharide group 7.5mg / kg, Group B is 15mg / kg of Cyber polysaccharide group, c group is 30mg / kg / kg of Cyber polysaccharide group, p group is 100mg / kg of Libelin, 10 in each group; Animal is anesthetized by propofol, and the N group drops of 1640 medium was 30 μl as a control; dripped infection 10LD 50 H1N1 virus 30 μL, A, and P group infected H1N1 after 2 hours, the N group was administered 0.5% CMC gas in the N group, as normal and viral control, administration once a day, seven days of continuous administration, stop The drug was observed in 14 days, and the amount of animals and the number of deaths were recorded daily, and the drug protection rate of the drug on severe flu infection with pneumonia mice was calculated;
[0047] The results show that after oral cork pol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com