Intracellular responsive ruthenium complex anticancer drug and preparation method thereof
A technology of ruthenium complexes and anticancer drugs, which is applied in the field of ruthenium complex anticancer drugs and its preparation, can solve the problems of fewer types and preparation methods, and achieve the effect of strong antitumor activity and enhanced binding ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
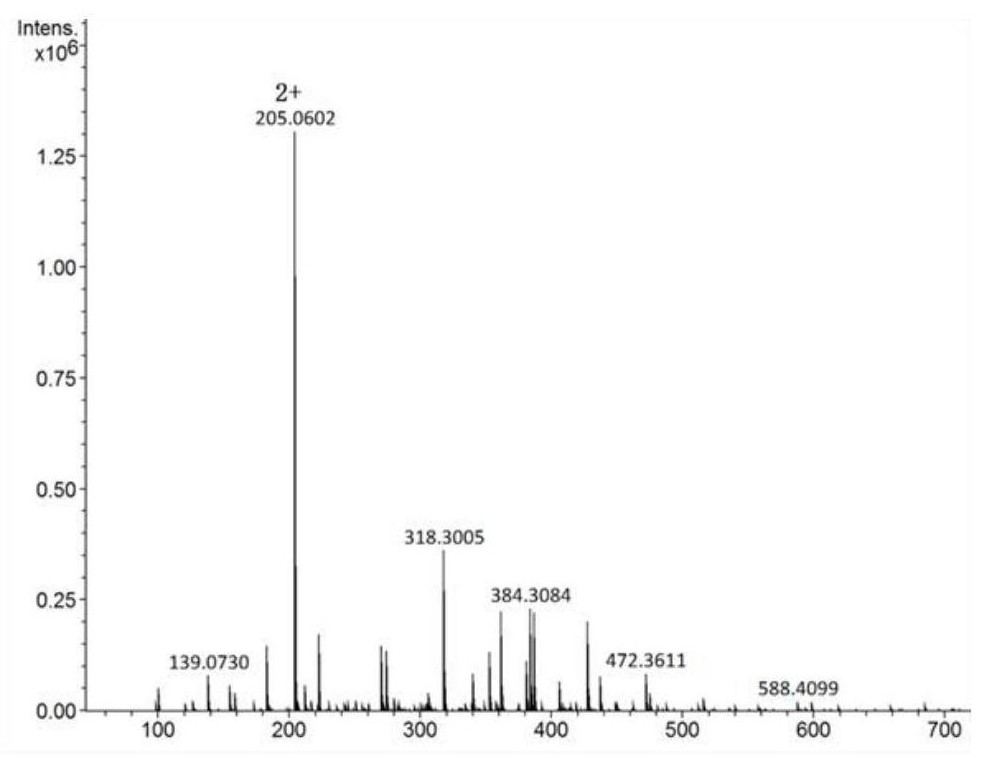
[0050] The synthesis of the ruthenium complex anticancer drug of embodiment 1 intracellular response
[0051] The synthesis method of the ruthenium complex anticancer drug that responds in the cell comprises:
[0052] (1) Synthesis of PN
[0053] Weigh 20 g of 1,10-phenanthroline and add it to 30 mL of concentrated sulfuric acid, stir slowly, heat the oil bath to 165 ° C, slowly add 120 mL of concentrated sulfuric acid and concentrated nitric acid mixture (volume ratio 1:1), and produce Large amounts of brown gas. After the dropwise addition, reflux for 3 hours, and naturally cool to room temperature to obtain a brownish-yellow liquid. Place it in an ice-water bath, slowly add 10mol / L ice NaOH solution dropwise, keep stirring, and adjust the pH to about 5. There is a large amount of A yellow precipitate was produced, which was filtered by suction and dried to obtain PN (5-nitro-1,10-phenanthroline).
[0054] (2) Synthesis of PNH
[0055] in N 2 Under ambient conditions, d...
Embodiment 2
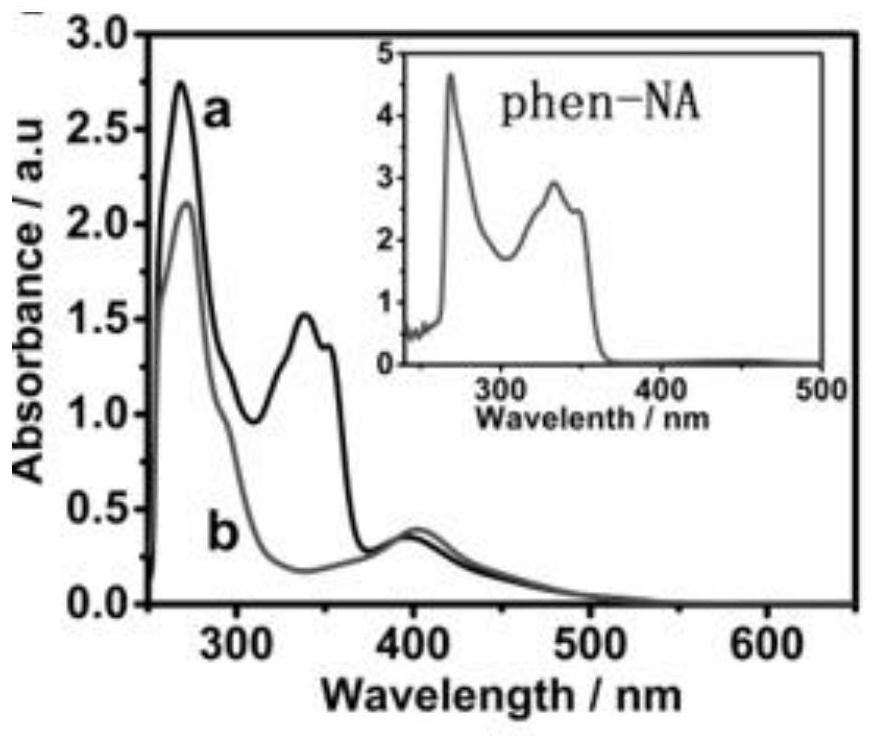
[0063] Kinetic experiment of embodiment 2 RuDPH and RuDPNI binding DNA in vitro
[0064] Experimental method: use different concentrations of DNA to titrate the UV-visible spectrum changes of RuDPNI and RuDPH, and calculate the binding constants of RuDPNI and RuDPH to DNA.
[0065] Experimental results: see image 3 , a of the figure shows that RuDPNI exhibits a color reduction effect (at 346 nm and 395 nm) and a red shift at 401 nm (about 6 nm) after adding increasing amounts of ct-DNA. RuDPH binds weakly to DNA (b), and no significant color reduction effect was observed in the figure. From the UV-Vis titration spectra, it was speculated that RuDPNI binds to DNA in an intercalation mode. From the absorbance at 346nm, the RuDPNI binding constant was calculated (see Figure 6 )K b The value is 1.204×10 6 m -1 , K of RuDPH b =8.497×10 4 m -1 . By modifying the 1,8-naphthalimide group, the DNA-binding affinity of RuDPNI was enhanced about 14.17-fold.
Embodiment 3
[0066] Example 3 In Vitro Cytotoxicity Test
[0067]Experimental method: The international general MTT method is used to analyze the toxicity of materials. The full name of MTT is 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide, ThiazolylBlueTetrazolium Bromide, Chinese is 3-(4,5-dimethylthiazolyl-2)- 2,5-Diphenyl tetrazolium bromide, trade name: thiazolium blue, is a yellow dye. MTT method is a method for detecting cell survival. The detection principle is that succinate dehydrogenase in the mitochondria of living cells can reduce exogenous MTT to water-insoluble blue-purple crystal formazan (Formazan) and deposit in the cells, while dead cells have no such function. Dimethyl sulfoxide (DMSO) can dissolve formazan in cells, and its light absorption value is measured at a wavelength of 540 or 720 nm with an enzyme-linked immunosorbent assay, which can indirectly reflect the number of living cells. Within a certain cell number range, the amount of MTT cryst...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com