Acinetobacter sp. ZJPH1806 and application thereof to preparation of miconazole chiral intermediate
A technology of bacillus and wet bacteria, which is applied in the field of preparation of miconazole chiral intermediates, can solve the problems of environmental pollution and low yield, and achieve the effect of high optical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1, screening and identification of Acinetobacter ZJPH1806 strain
[0037] 1. Strain screening
[0038] 1) Enrichment culture and primary screening: Add 1g of fresh soil samples collected near the Jianfeng Mountain Orchard in Wucheng District, Jinhua City, Zhejiang Province, into a 250mL shake flask filled with 50mL enrichment medium, culture at 30°C and 200rpm for 5 days , transfer 1 mL of the culture solution to a fresh enrichment medium, continue the enrichment culture for 5 days, and repeat the enrichment twice. The enrichment medium consists of: (NH 4 ) 2 SO 4 2g / L, KH 2 PO 4 2g / L, NaCl1g / L, MgSO 4 ·7H 2 O 0.5g / L, 2,2',4'-trichloroacetophenone (2g / L) is the only carbon source, the solvent is water, pH 6.5.
[0039] 2) Plate culture: Dilute the enriched culture solution with normal saline for 10 4 -10 6 After doubling, it was spread on a plate containing screening medium, cultured at 30°C for 2 days, and a single colony strain was obtained after...
Embodiment 2
[0060] Embodiment 2 optimizes medium composition
[0061] 1. Optimize the types of carbon sources, nitrogen sources and inorganic salts in the fermentation medium
[0062] Fermentation medium composition: carbon source 15g / L, nitrogen source nitrogen content 1.8g / L, inorganic salt 1mM, solvent is water, pH 6.5.
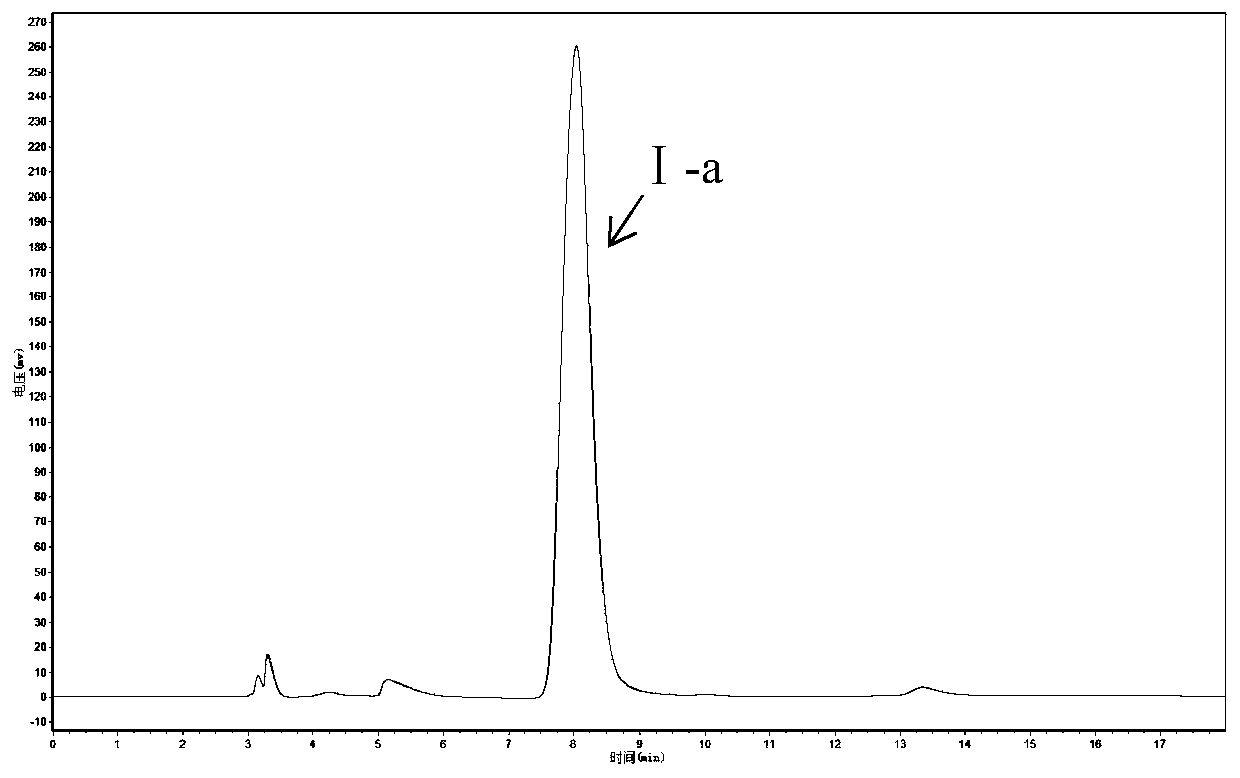
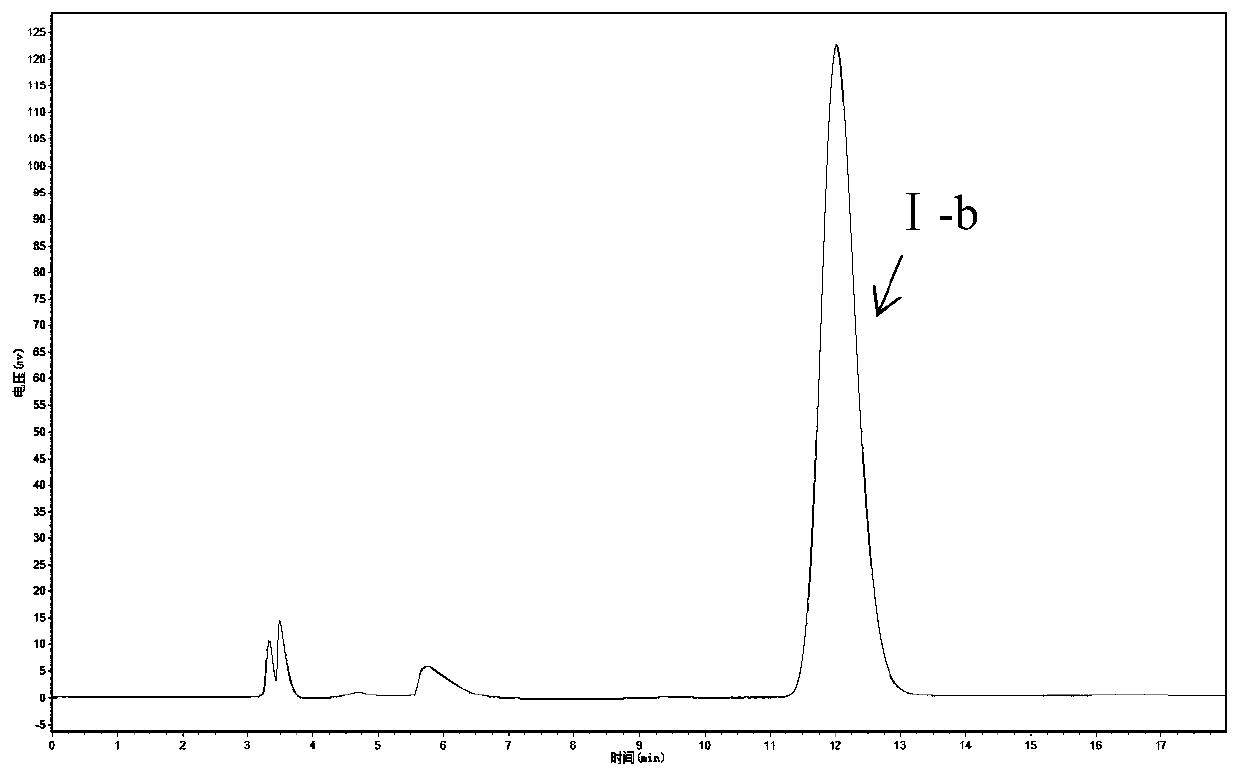
[0063] Acinetobacter ZJPH1806 was inoculated into fermentation media composed of different carbon sources, nitrogen sources and inorganic salts. The culture conditions were as follows: initial pH value 6.5, shake flask filling volume 100mL / 250mL Erlenmeyer flask, culture temperature 30°C, shaker The rotating speed is 200rpm, the inoculum volume is 10%, and the incubation time is 24h. The enzyme activity and ee value are determined by the method in Example 1.
[0064] By examining the single factor experiments (shown in Table 1-Table 3 and Table 5) of different types of carbon sources, nitrogen sources and inorganic salts, the best carbon sources, nitrogen sources and...
Embodiment 3
[0087] Embodiment 3: the acquisition of wet fungus cells
[0088] (1) Incline culture: Acinetobacter ZJPH1806 was inoculated into the slant medium, cultured in a constant temperature incubator at 30°C for 1-2 days to obtain slant strains, and stored in a refrigerator at 4°C. The final concentration of the slant medium consists of: glucose 15g / L, peptone 7.5g / L, yeast extract 6g / L, (NH 4 ) 2 SO 4 3g / L, KH 2 PO 4 1.5g / L, NaCl 0.75g / L, MgSO 4 ·7H 2 O 0.75g / L, agar powder 20g / L, solvent is water, pH 6.5.
[0089] (2) Seed culture: Inoculate the slant bacteria into the seed medium, cultivate at 30°C and 200rpm for 12 hours, and obtain the seed liquid; the composition of the seed medium is: glucose 15g / L, peptone 20g / L, yeast extract 10g / L, (NH 4 ) 2 SO 4 2g / L, KH 2 PO 4 2g / L, NaCl1g / L, MgSO 4 ·7H 2 O 0.5g / L, solvent is water, pH 6.5.
[0090] (3) Fermentation culture: transfer the seed liquid to the fermentation medium with an inoculum size of 10% (v / v), culture a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com