Method for evaluating quality of Herba Cirsii Setosi through quantitative analysis of multicomponents by single marker
A small thistle and quality technology, applied in measuring devices, instruments, scientific instruments, etc., can solve the problems of many varieties of standard products, complex content determination methods, and high detection economic costs, achieve high detection sensitivity, and reduce detection costs and detection time. , The effect of simplifying the quantitative detection method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
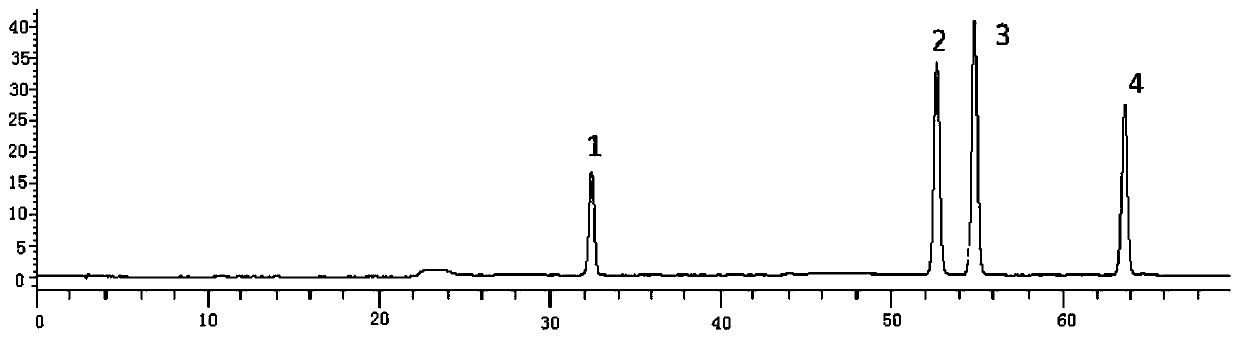
[0050] The multi-component detection method of thistle in the present invention adopts the method of one test and multiple evaluations, and uses rutin as an internal reference to measure the contents of succulentin, luteolin and apigenin in thistle, specifically comprising the following steps:
[0051] Step 1, preparation of reference substance standard solution
[0052] Step 1.1. Accurately weigh the rutin standard, mongoside standard, luteolin standard, and apigenin standard, respectively, and place them in 10mL measuring bottles, add methanol to dissolve, and set the volume to the mark to obtain a concentration of 0.50mg The rutin standard solution in / mL, the mongoside standard solution with a concentration of 0.05mg / mL, the luteolin standard solution with a concentration of 0.51mg / mL and the apigenin standard solution with a concentration of 0.41mg / mL;
[0053] Step 1.2. Take 1 mL each of the above-mentioned rutin, mongoside, luteolin and apigenin standard solution respec...
Embodiment 2
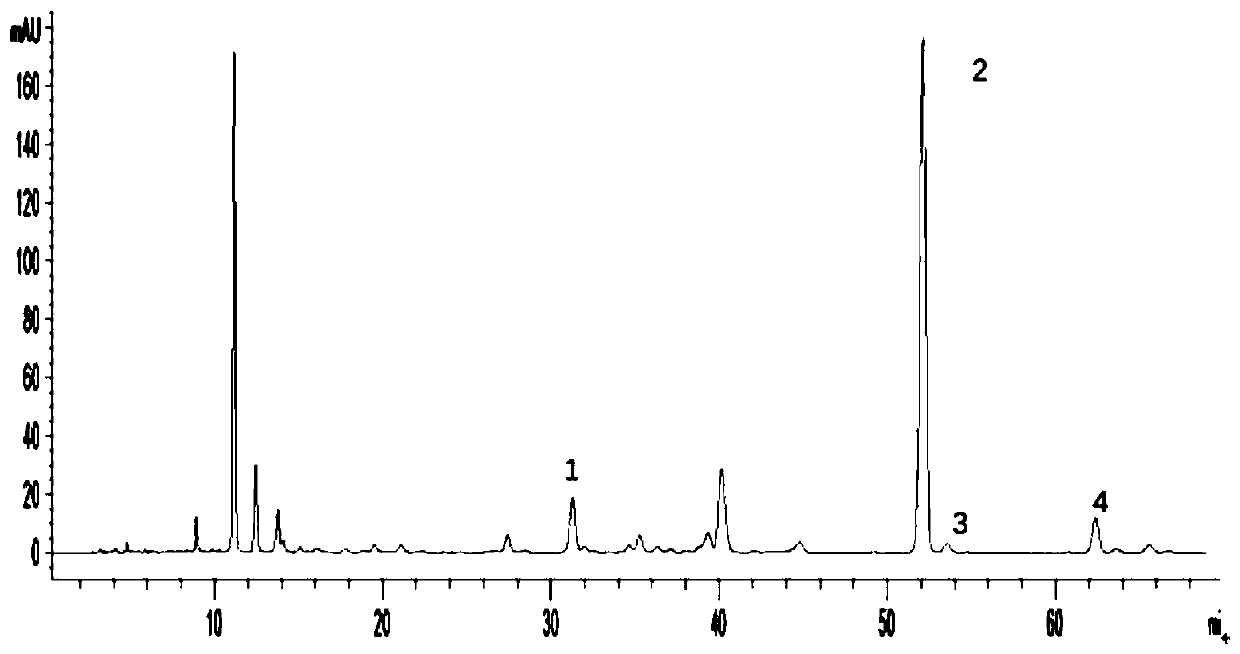
[0091] The multi-component detection method of thistle in the present invention adopts the method of one test and multiple evaluations, and uses rutin as an internal reference to measure the contents of succulentin, luteolin and apigenin in thistle, specifically comprising the following steps:
[0092] Step 1, preparation of reference substance standard solution
[0093] Step 1.1. Accurately weigh the rutin standard, mongoside standard, luteolin standard, and apigenin standard, respectively, and place them in 10mL measuring bottles, add methanol to dissolve, and set the volume to the mark to obtain a concentration of 0.50mg The rutin standard solution in / mL, the mongoside standard solution with a concentration of 0.05mg / mL, the luteolin standard solution with a concentration of 0.51mg / mL and the apigenin standard solution with a concentration of 0.41mg / mL;
[0094] Step 1.2. Take 1 mL each of the above-mentioned rutin, mongoside, luteolin and apigenin standard solution respec...
Embodiment 3
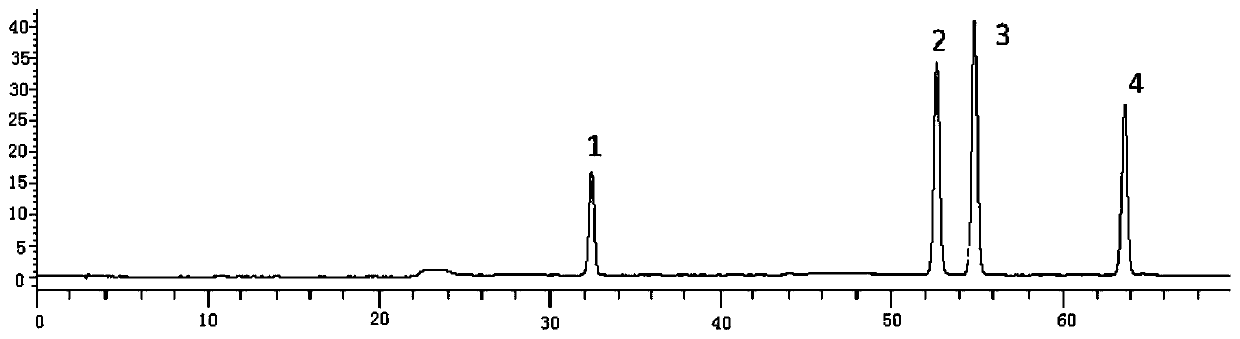
[0119] The multi-component detection method of thistle in the present invention adopts the method of one test and multiple evaluations, and uses rutin as an internal reference to measure the contents of succulentin, luteolin and apigenin in thistle, specifically comprising the following steps:
[0120] Step 1, preparation of reference substance standard solution
[0121] Step 1.1, accurately weigh the standard products of rutin, mongoside, luteolin and apigenin, place them in 10mL measuring bottles respectively, add methanol to dissolve, set the volume to the mark, and obtain rutin with a concentration of 0.50mg / mL Standard solution, a concentration of 0.05mg / mL of the standard solution of mongoside, a concentration of luteolin standard solution of 0.51mg / mL and a concentration of 0.41mg / mL of apigenin standard solution;
[0122] Step 1.2. Take 1 mL each of the above-mentioned rutin, mongoside, luteolin and apigenin standard solution respectively, put them in a 10 mL volumetri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com