Calibration product and quality control product for mass spectrometry detection of vitamin D and metabolite thereof, and preparation method and applications thereof
A technology for mass spectrometry detection and vitamins, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of inability to meet clinical precision measurement, large accuracy deviation, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0120] The preparation (1) of the mass spectrometry detection of embodiment 1 vitamin D metabolites
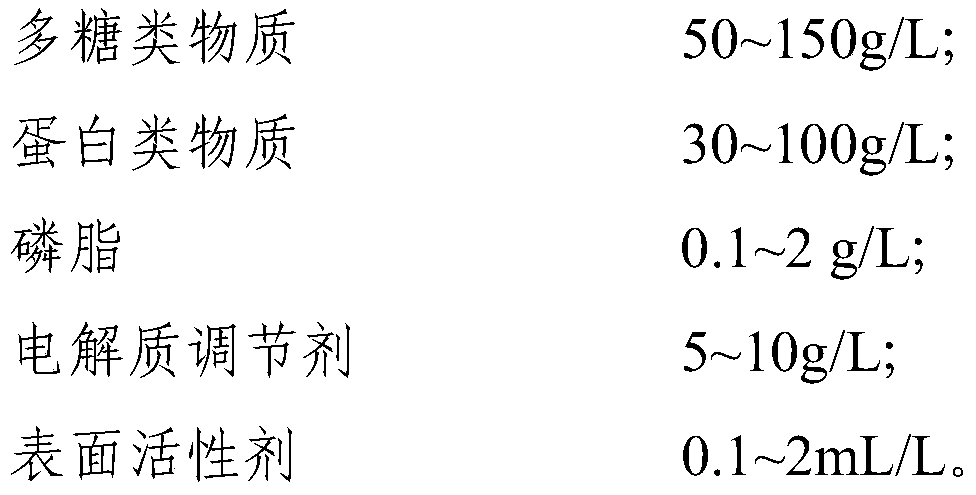
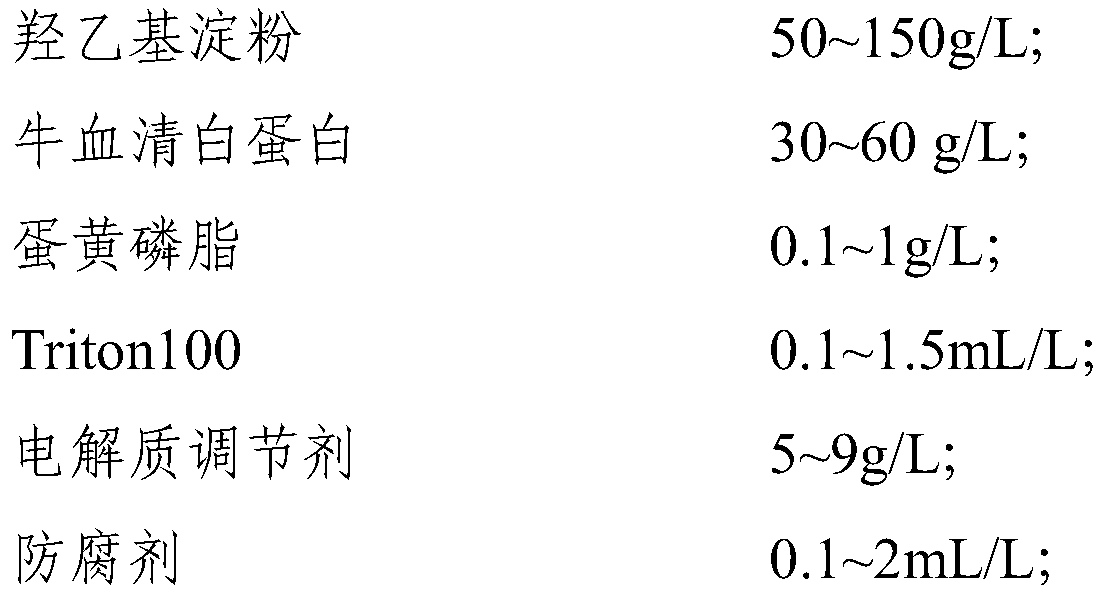
[0121] This embodiment provides a calibrator for mass spectrometry detection of vitamin D metabolites. The formula for preparing the matrix of the calibrator is as follows: 50 g / L of hydroxyethyl starch, 50 g / L of bovine serum albumin, and 0.5 g / L of egg yolk phospholipids , Triton100 0.5mL / L, sodium chloride 9g / L, Proclin300 0.5mL / L, natamycin 0.5mL / L.
[0122] The calibrator consisted of the matrix described above and various concentrations of vitamin D metabolites.
[0123] This embodiment provides the preparation method (1L volume) of the calibrator for the mass spectrometry detection of vitamin D metabolites, specifically as follows:
[0124] 1. Preparation of blank matrix
[0125] (1) Preparation of solution A: Accurately absorb 0.5ml of Proclin300 and 0.5ml of natamycin, dissolve them in 50ml of double distilled water, and mix well. Accurately weigh 9g of sodium chlo...
Embodiment 2
[0142] Example 2 Preparation of quality control product for vitamin D metabolite mass spectrometry detection (1)
[0143] 1. Preparation of vitamin D metabolites quality control level 1 and quality control level 2
[0144] The preparation of the blank matrix and high-value solution used for the preparation of quality control products is the same as that in Example 1.
[0145] Mix 100ml of the high-value solution and 800ml of the blank matrix solution to obtain a quality control level 1 (quality control L1) solution.
[0146] Mix 100ml of the high-value solution and 500ml of the blank matrix solution to obtain a quality control level 2 (quality control L2) solution.
[0147] The above solution is divided into 5ml medical brown glass bottles, and the filling volume is 3ml. Perform lyophilization. The freeze-drying process is a conventional freeze-drying process. During the freeze-drying process, the maximum temperature for heating is 37°C. After freeze-drying, semi-finished ...
experiment example 1
[0160] Experimental Example 1 Effect of quality control and calibrator on matrix effect in the detection of vitamin D metabolites
[0161] The evaluation method of matrix effect is a routine method in this field, and a brief description is as follows:
[0162] 1. Materials
[0163] 1.1. Reagents, calibrators and instruments evaluated
[0164] 1.2. Control reagents, calibrators and instruments
[0165] 1.3. Processed sample: it is the object to be evaluated in this experimental example (the calibrator prepared in Example 1 and the quality control product prepared in Example 2).
[0166] 1.4. Fresh human serum samples
[0167] At least 20 cases of fresh human serum samples, the concentration of serum should cover the linear range.
[0168] 2. Operation
[0169] 2.1. Prepare samples
[0170] Prepare samples according to the requirements of 1.
[0171] 2.2. Analysis of the evaluated method
[0172] The processed samples were spiked in 20 cases of fresh human sera and assay...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com