Application of High Speed Countercurrent Chromatography in Biotransformation of Glycosides
A high-speed counter-current chromatography and glycoside technology, applied in the field of glycoside conversion, can solve the problems of undisclosed high-speed counter-current chromatography, cumbersome steps, etc., and achieve the effects of eliminating product inhibition, improving reaction efficiency, and maintaining activity and concentration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
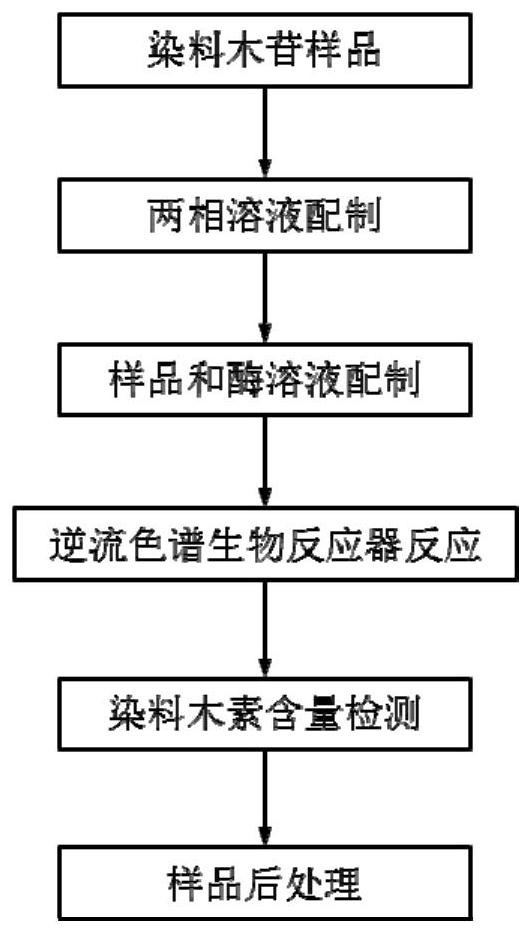
[0066] The biotransformation of embodiment 1 genistin
[0067] 1. Preparation of two-phase solvent and sample solution
[0068] Put the ethyl acetate / buffer solution (1:1, v / v) solvent system in the separatory funnel according to the ratio, shake it fully, let it stand for stratification, and wait for the two phases to reach equilibrium, take the upper and lower phases respectively, and ultrasonically remove them. Reserve after gas.
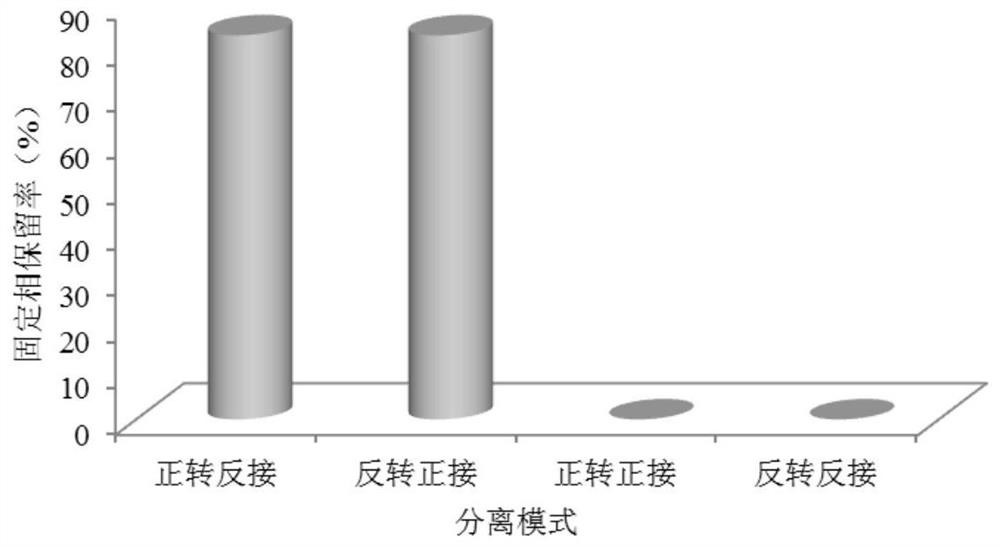
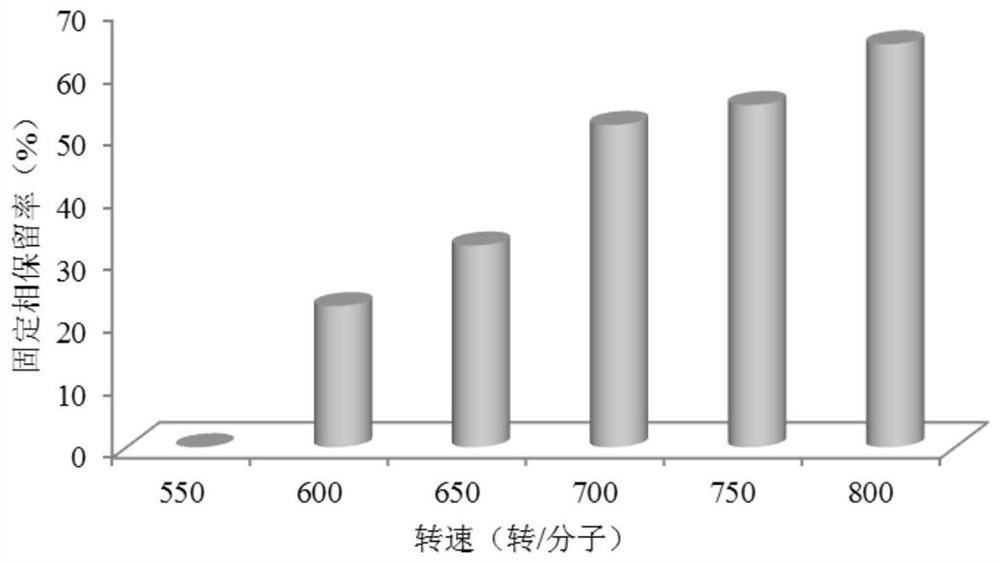
[0069] Configure the following three solutions according to the requirements of the countercurrent chromatography bioreactor: (1) Take part of the upper phase solution as the mobile phase of the countercurrent chromatography, add genistin, and ultrasonically dissolve it fully to prepare a 62.5mg / L substrate solution (2) The lower phase is a stationary phase, add β-glucosidase, fully mix, and be mixed with a 0.75 mg / mL bioreactor enzyme solution; (3) get part of the upper phase solution as a blank mobile phase, and set aside. The above three sol...
Embodiment 2
[0080] The biotransformation of embodiment 2 polydatin
[0081] 1. Solvent system selection
[0082] According to the conversion of polydatin, the following two-phase solvent system was selected for the countercurrent chromatographic enzyme reactor in this example, namely n-hexane / buffer solution (1:1, v / v), ethyl acetate / buffer solution (1:1 , v / v), chloroform / buffer solution (1:1, v / v) and n-butanol / buffer solution (1:1, v / v) solvent system, using HPLC method to determine polydatin (substrate) and resveratilla Partition coefficient (K D ) value, the method is as follows: according to the proportion of the solvent system, prepare 20mL solvent, shake fully, and let stand to separate layers. First take 5 mL of the lower phase in a test tube, add about 1 mg of polydatin and resveratrol samples, and measure the peak area of each target peak in the lower phase by HPLC, denoted as A 1 , and then add an equal volume of the upper phase, fully shake, let stand for stratification,...
Embodiment 3
[0098] The conversion of embodiment 3 daidzein
[0099] 1. Solvent system selection
[0100] For the conversion of daidzein, the following two-phase solvent system was selected for the countercurrent chromatographic enzyme reactor in this example, that is, n-hexane / buffer solution (1:1, v / v), ethyl acetate / buffer solution (1:1 , v / v), chloroform / buffer solution (1:1, v / v) and n-butanol / buffer solution (1:1, v / v) solvent system, using HPLC method to determine total soybean isoflavone glycosides (substrate) and the partition coefficient (K D ) value, the method is as follows: according to the proportion of the solvent system, prepare 20mL solvent, shake fully, and let stand to separate layers. First take 5 mL of the lower phase in a test tube, add about 1 mg of each of the substrate and the product, and use HPLC to measure the peak area of each target peak in the lower phase, which is recorded as A 1 , and then add an equal volume of the upper phase, fully shake, let stand ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com