Method for synthesizing tadalafil
A technology for tadalafil and compounds, applied in the field of preparation of tadalafil, can solve the problems of controlled starting materials, complex preparation process, difficult industrial production, etc., achieving short synthesis steps, simple process operation, and short production cycle Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Weigh 200g of compound of formula 1 (1.02mol) and 204.2g of compound of formula 2 (1.0mol) into a 2L three-neck flask, stir, add 1500mL of toluene and 228g of trifluoroacetic acid (2.0mol), and heat to 110°C. React until the compound of formula 2 is less than 1.0%, lower the temperature to 10 degrees Celsius, filter with suction, and dry under reduced pressure at 60 degrees Celsius to obtain 286g of compound 3. The molar yield relative to the compound of formula 2 is 85%, and the purity is 98.5%. 1H NMR (600 MHz, [D6]DMSO): δ = 3.13 (m,1H), 3.37 (dd, J =12.0, 4.4 Hz, 1 H), 4.56 (dd, J = 11.7, 4.7 Hz,1 H), 5.83 (s, 1 H), 6.08 (m,2 H), 6.96 (s, 1 H), 7.03–7.05 (m, 3H), 7.12 (t, J = 7.3 Hz, 1 H), 7.29 (d, J = 8.2 Hz, 1 H), 7.54 (d, J = 7.9 Hz, 1 H) , 10.12 (br, 2 H), 10.80 (s, 1 H). MS(m / z):335.1[M-H] - .
Embodiment 2
[0031] Put 200g of the compound of formula 3 (0.59mol) in a 3L three-necked flask, add 2000mL of dichloromethane and 137.3g of triethylamine (1.36mol), protect with nitrogen after replacing the air, keep stirring, cool down to 0 degrees Celsius, and control Add 146g of chloroacetyl chloride when the temperature of the system does not exceed 10 degrees Celsius. After reacting for 1 hour, add 500g of water slowly, separate layers, wash the dichloromethane phase with 500g of water once, concentrate to dryness under reduced pressure, add 1000g of absolute ethanol, and stir for 4 hour, suction filtration, and drying under reduced pressure at 60°C to obtain 219g of the compound of formula 4, with a molar yield of 90% and a purity of 99.0%.
[0032] 1H NMR (400 MHz, [D6]DMSO): δ = 2.96-3.02 (m,1H), 3.40-3.46 (m,2H),4.39 (d, J = 14 Hz,1 H) , 4.81 (d, J = 14 Hz,1 H), 5.04 (d, J = 6.4 Hz,1 H) ,5.94 (d, J = 11.2 Hz,1 H),6.59-6.75 (m, 3 H),6.99–7.08 (m, 2H), 7.25 (d, J =7.6 Hz, 1 H)...
Embodiment 3
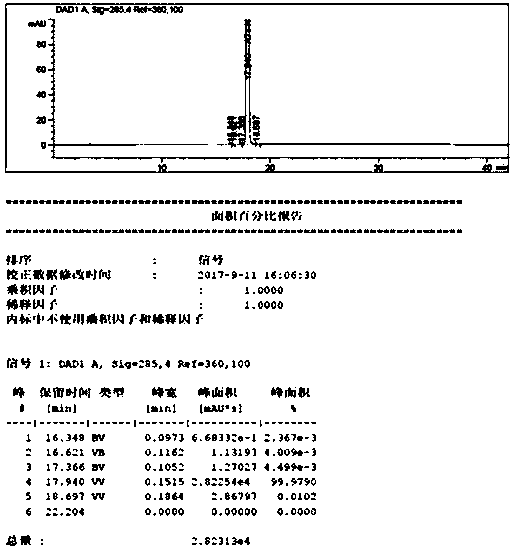
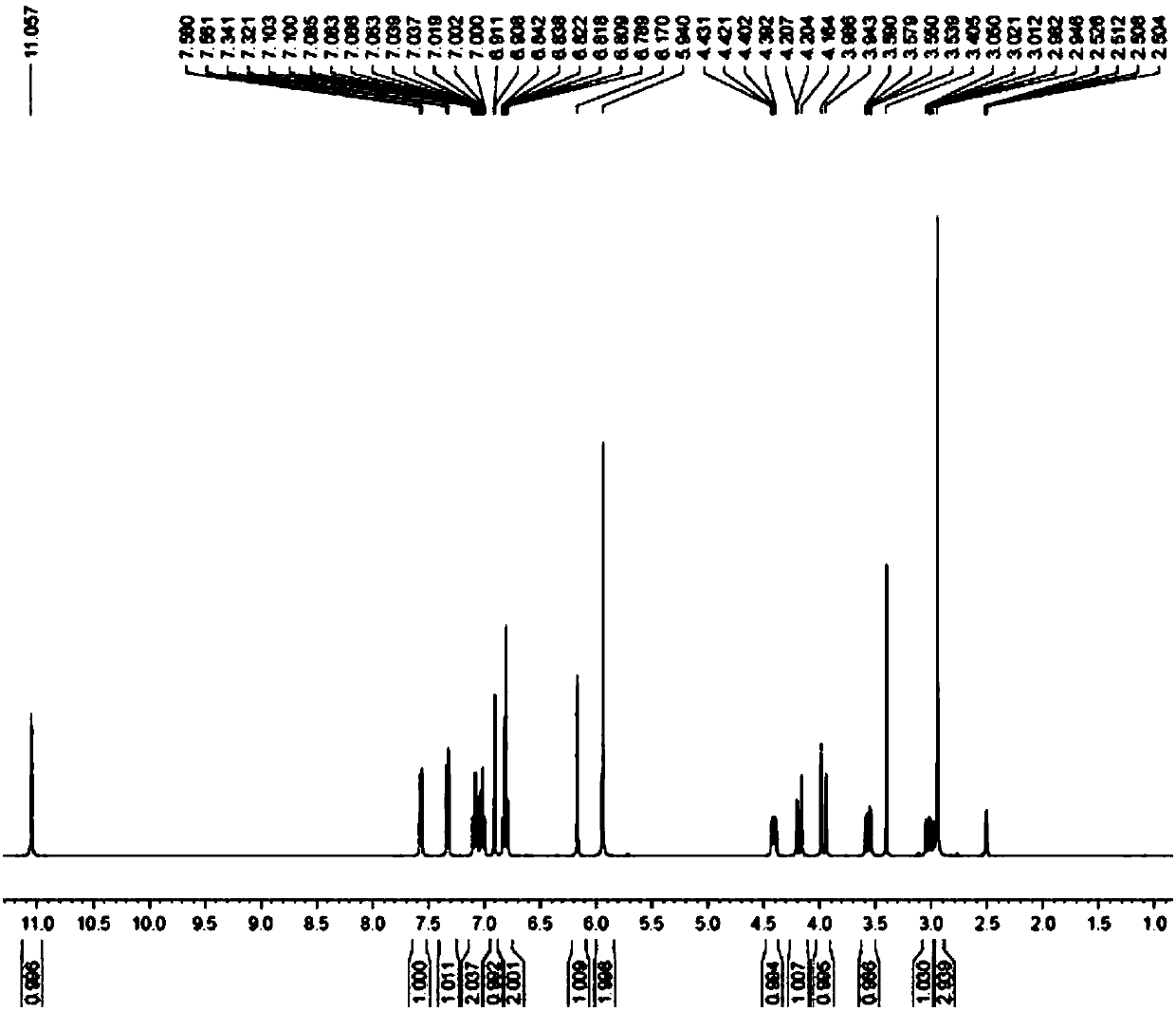
[0034] Put 100g of the compound of formula 4 (0.24mol) in a 1L three-necked flask, add 400mL of tetrahydrofuran and 75.2g of 40% aqueous methylamine (0.97mol), stir, heat up to 50 degrees Celsius, react until the compound of formula 4 disappears, add 500ml of Water alcohol, cooled to 0 degrees Celsius, suction filtered, dried under reduced pressure at 60 degrees Celsius to obtain 85 g of tadalafil, molar yield: 91%, purity 99.9%. 1HNMR (CDCl3): see attached figure 2 ; MS(m / z): 390.1[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com