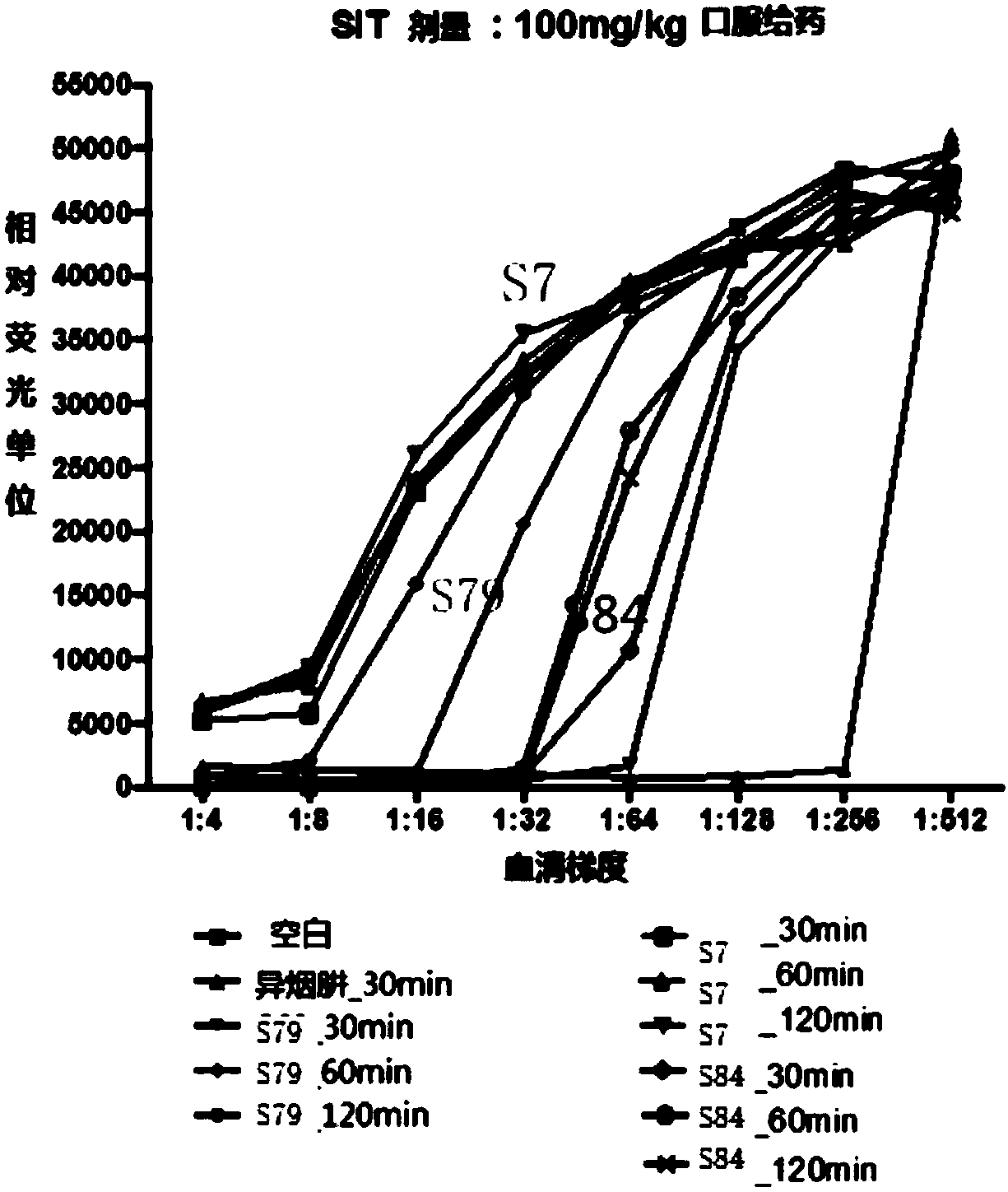
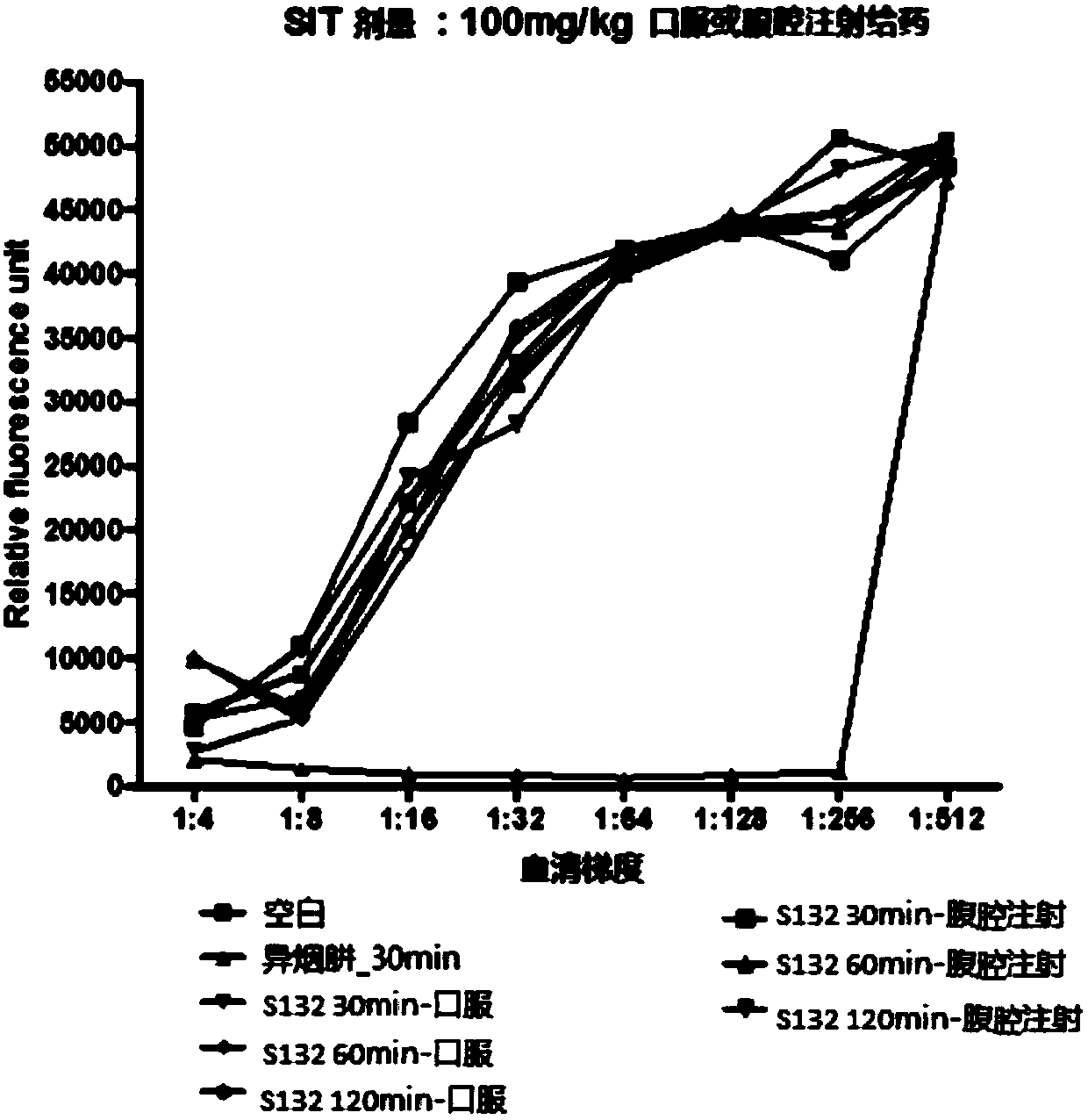
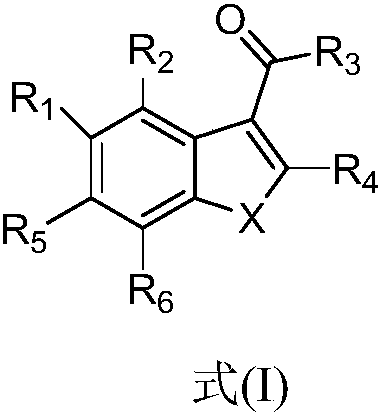
Benzofuran and benzofuran coumarin derivatives and preparation method and application thereof
A technology for benzofuran and derivatives, which is applied in the field of preparation of benzofuran and Coumestans derivatives, can solve the problems of aggravating the burden of patients, reducing the curative effect of tuberculosis, prolonging the treatment period, etc., and achieves good bacteriostatic activity and good bioavailability. degree of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Example 1 Preparation of 5-hydroxyl-(4-(1-(4-methylpiperazinyl))methylene)-2-phenylbenzofuran-3-carboxylic acid ethyl ester (compound S1)
[0089]1.1 Add 3.45g of diethyl carbonate and 0.87g of sodium hydride to 15mL of toluene, then slowly add 2.19g of acetophenone dropwise to the system under the protection of nitrogen, stir at 110°C for 30min, after the reaction is completed, cool to room temperature and add ice water , add glacial acetic acid until the system is neutral, extract with ethyl acetate (50mL×3), separate the organic layer, wash with saturated brine 100ml, dry over anhydrous sodium sulfate, evaporate the solvent ethyl acetate, and the crude product is subjected to flash column chromatography ( Petroleum ether: ethyl acetate = 15:1) to obtain ethyl benzoyl acetate (compound 1‐1);
[0090] 1.2 Dissolve 1.38g of ethyl benzoyl acetate and 86.37mg of copper trifluoromethanesulfonate in 20mL of toluene, slowly add 0.52g of 1,4‐benzoquinone dropwise under nitrog...
Embodiment 2
[0093] Example 2 Preparation of 4-(dipropylamino)methylene-5-hydroxyl-2-phenylbenzofuran-3-carboxylic acid ethyl ester (compound S2)
[0094] Prepared by a method similar to Example 1, the difference is that N-methylpiperazine is replaced by dipropylamine, and the rest of the required raw materials, reagents and preparation methods are the same as in Example 1 to obtain the final product. 1 H NMR (400MHz, CDCl 3 )δ7.78–7.72(m,2H),7.48–7.40(m,3H),7.31(d,J=8.8Hz,1H),6.85(d,J=8.8Hz,1H),4.35(q,J =7.1Hz, 2H), 4.06(s, 2H), 2.54(t, J=7.5Hz, 4H), 1.70– 1.51(m, 4H), 1.29(t, J=7.2Hz, 3H), 0.91(t ,J=7.4Hz,6H). 13 C NMR (101MHz, CDCl 3 )δ166.4, 156.6, 155.6, 148.1, 130.0, 129.5, 128.3, 127.8, 125.3, 115.2, 112.9, 110.6, 110.0, 61.4, 55.7, 54.2, 19.6, 13.9, 11.8. M+H) + 396.2169; Found 396.2168.
Embodiment 3
[0095] Example 3 5-hydroxyl-(4-(1-(4-(4-(hydroxymethyl) piperidinyl)) methylene)-2-phenylbenzofuran-3-carboxylate ethyl ester (compound S3) preparation
[0096] Prepared by a method similar to Example 1, except that 4-hydroxymethylpiperidine is used instead of N-methylpiperazine, and the rest of the required raw materials, reagents and preparation methods are the same as in Example 1 to obtain the final product. 1 H NMR (400MHz,MeOD)δ7.73–7.67(m,2H),7.51–7.45(m,3H),7.39(d,J=8.9Hz,1H),6.88(d,J=8.9Hz,1H) ,4.33(q,J=7.1Hz,2H),4.16(s,2H),3.43(d,J=6.3Hz,2H),3.33–3.30(m,1H),3.19(d,J=11.9Hz, 2H), 2.45(t, J=11.3Hz, 2H), 1.84(d, J=13.0Hz, 2H), 1.70–1.56(m, 1H), 1.45–1.30(m, 2H), 1.23(t,J =7.1Hz, 3H). 13 C NMR (101MHz, MeOD) δ167.6, 159.3, 156.1, 149.7, 131.2, 130.9, 129.5, 129.2, 127.2, 115.8, 112.7, 112.5, 111.4, 67.3, 64.3, 62.7, 56.5, 53.8, 38.9, 249.2, MS (ESI)m / z: Calcd for C24H28NO5(M+H) + 410.1962; Found 410.1980.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com