Monoterpene phenolic derivative, synthetic method of monoterpene phenolic derivative and application of monoterpene phenolic derivative in pesticides
A synthesis method and monoterpene phenol technology are applied in the field of pesticides and achieve the effects of good bactericidal effect, simple structure and easy synthesis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
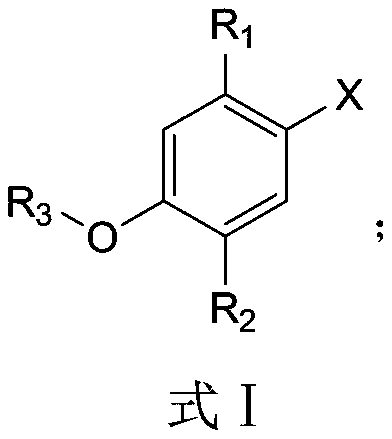
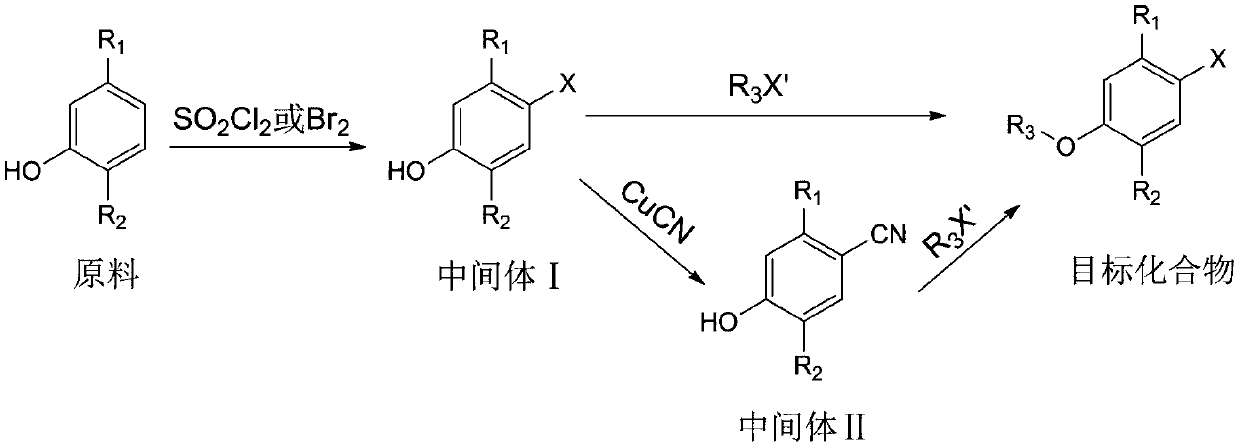
[0033] Its synthetic steps of the synthetic method of monoterpene phenol compound of the present invention are as follows:
[0034]
[0035] The application of the monoterpene phenolic compound of the present invention in pesticides is used to inhibit or kill phytopathogenic fungi, and the phytopathogenic fungi that are inhibited or killed are tomato early blight, tomato cinerea, cucumber fusarium wilt, and rice blast fungus or rice sheath blight.
Embodiment 1
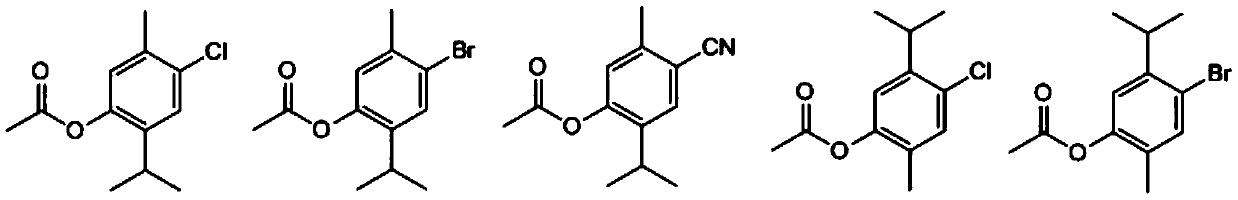
[0036] Embodiment 1: monoterpene phenolic compound (C 12 h 15 ClO 2 )Synthesis.
[0037] The concrete steps of the synthetic method of this monoterpene phenol compound are as follows:
[0038] (1) Synthesis of intermediate 5-methyl-2-isopropyl-4-chlorophenol
[0039] 1.5 g of thymol was dissolved in 20 mL of dichloromethane, and the resulting solution was cooled to 0°C. A solution of 1.6 g of sulfonyl chloride in dichloromethane (5 mL) was slowly added within 20 min using a constant pressure dropping funnel, and the resulting mixture was continued to react at room temperature after the addition was complete. TLC monitors the progress of the reaction. After the reaction is completed, add 30 mL of distilled water, extract three times with 30 mL of ethyl acetate, combine the organic phases, dry and concentrate, and use eluent (petroleum ether: ethyl acetate=50:1) column chromatography to obtain the compound 5-Methyl-2-isopropyl-4-chlorophenol, yield 78%, pale yellow oil. ...
Embodiment 2
[0050] Embodiment 2: monoterpene phenolic compound (C 12 h 15 BrO 2 )Synthesis.
[0051] The concrete steps of the synthetic method of this monoterpene phenol compound are as follows:
[0052] (1) Synthesis of intermediate 5-methyl-2-isopropyl-4-bromophenol
[0053] 1.218 g of thymol was dissolved in 20 mL of acetic acid, and the resulting solution was cooled to 0°C. Add 0.51 mL of Br slowly within 20 min using a constant pressure dropping funnel 2 , After the dropwise addition was completed, the resulting mixture was stirred at room temperature for 6 h. The reaction was poured into 50 mL of ice water, extracted three times with 30 mL of dichloromethane, the organic phases were combined, dried and concentrated, and the intermediate 5-methanol was obtained by column chromatography with eluent (petroleum ether: ethyl acetate=40:1). 2-isopropyl-4-bromophenol, yield 82%, pale yellow oil.
[0054] (2) Synthesis of the target compound
[0055] Take 229mg of the intermediat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com