Carboxylic acid betaine type fluorine-containing compound as well as synthesis method and application thereof
A technology of carboxybetaine and synthesis method, which is applied in the directions of cyanide reaction preparation, chemical instruments and methods, preparation of organic compounds, etc., can solve problems such as difficult quantitative detection and quantitative research, and achieve high yield, Easy separation, simple purification method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
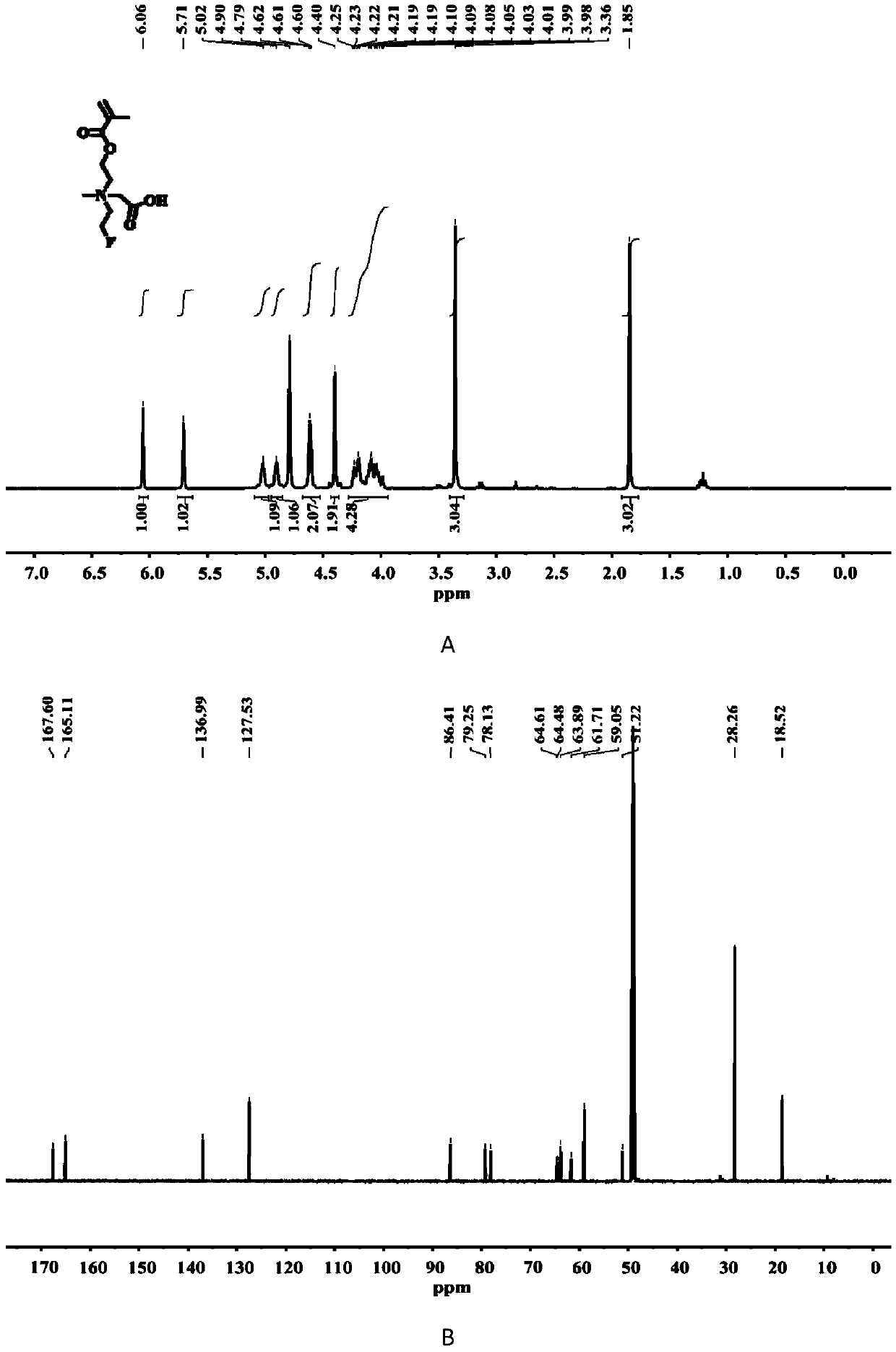
[0024] The synthesis of embodiment 1 compound C1
[0025] See the applicant's application on the same day, titled "Fluorinated tertiary amine compound and its synthesis method and its application" to obtain the starting tertiary amine compound, wherein, the method for newly synthesizing the fluorinated secondary amine compound is detailed in the applicant's application on the same day , named: fluorinated secondary amine compound and its synthesis method and application.
[0026] The chemical structure of compound C1 is shown in the following formula. The specific synthesis steps are as follows: First, 2-((2-fluoroethyl)methylamino)ethyl methacrylate (10 mmol), tert-butyl bromoacetate (1.5 eq.) were dissolved in anhydrous acetonitrile, and Under air protection, at 50 o C in a constant temperature oil bath for 24 h. After the reaction, the solvent was distilled off under reduced pressure and separated by column chromatography, using the DCM (dichloromethane) / methanol mixture...
Embodiment 2
[0029] Example 2 Synthesis of Compound C2
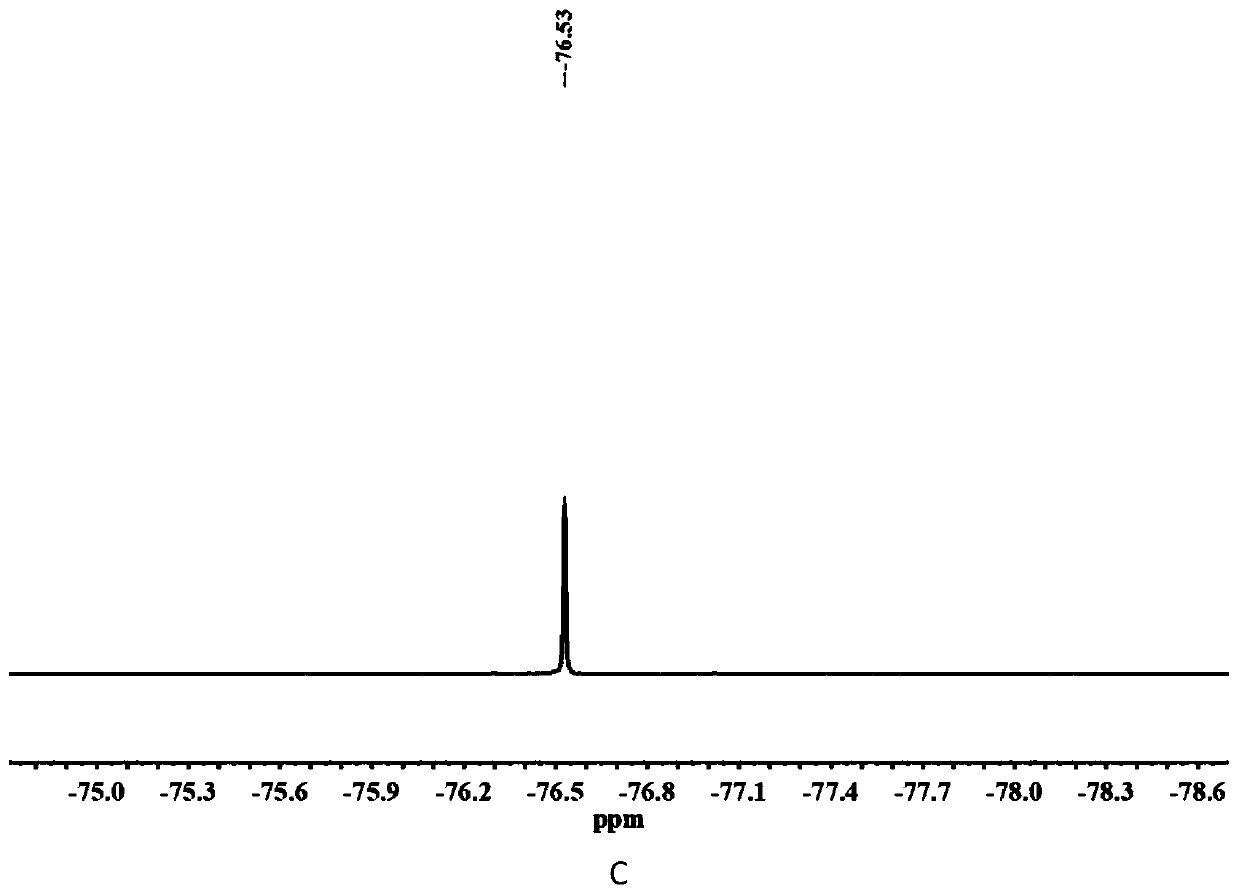
[0030] The chemical structure of compound C2 is shown in the following formula. The specific synthesis steps are as follows: First, 2-((2,2-difluoroethyl)methylamino)ethyl methacrylate (10 mmol), tert-butyl bromoacetate (1.5 eq.) were dissolved in anhydrous acetonitrile , under argon protection, at 50 o C in a constant temperature oil bath for 24 h. After the reaction, the solvent was distilled off under reduced pressure and separated by column chromatography. The eluent containing the target compound was collected with the DCM / methanol mixture as the eluent. After removing the solvent, the quaternary ammonium salt protected by tert-butyl group was obtained. product. Further, the tert-butyl protected quaternary ammonium salt product (1.0 eq.), triethylsilane (2.5 eq.) was dissolved in anhydrous DCM, and trifluoroacetic acid (13 eq.) was added dropwise under ice bath conditions, After returning to room temperature, the reaction wa...
Embodiment 3
[0033] Synthesis of Example 3 Compound C3
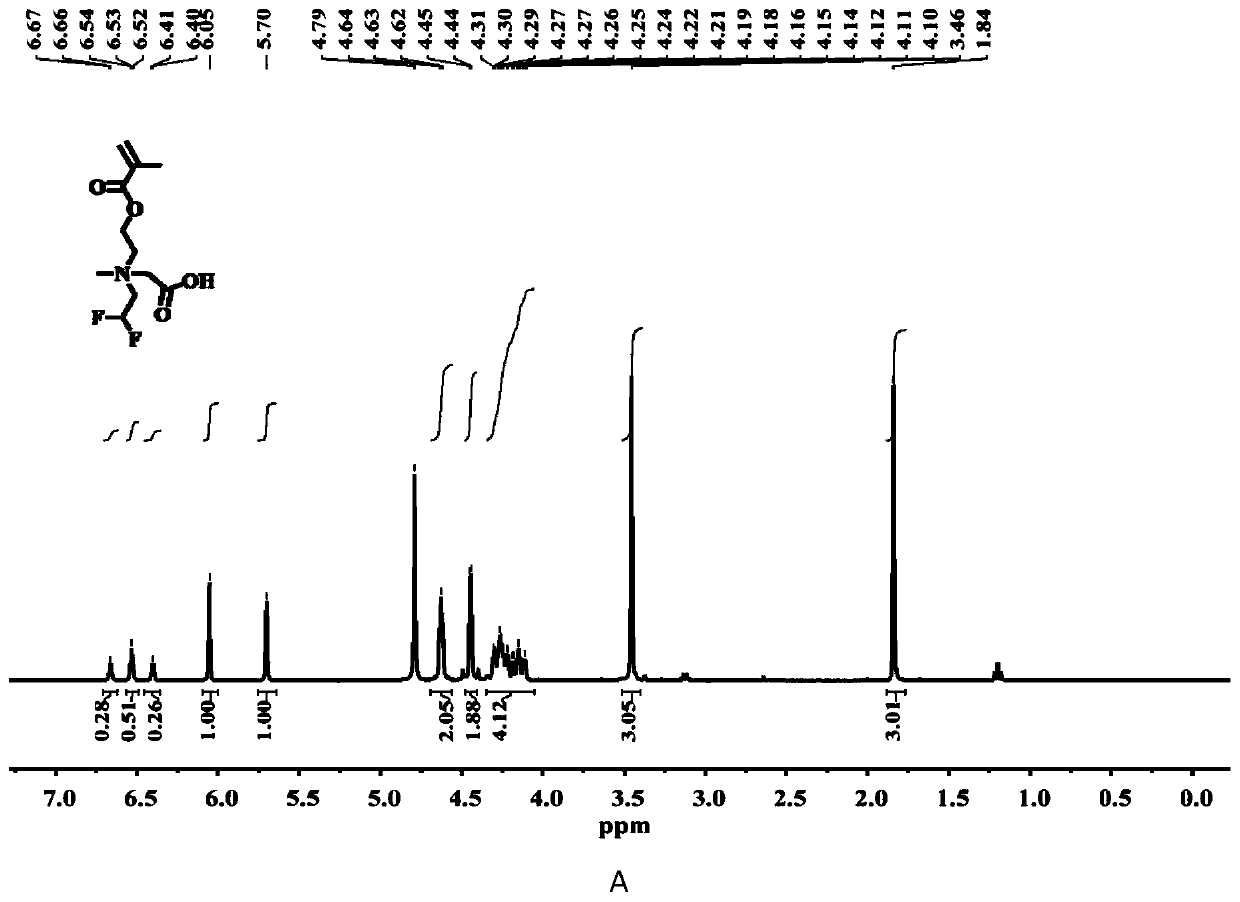
[0034] The chemical structure of compound C3 is shown in the following formula. The specific synthesis steps are as follows: First, 2-((3,3,3-trifluoropropyl)methylamino)ethyl methacrylate (10 mmol), tert-butyl bromoacetate (1.5 eq.) were dissolved in anhydrous In acetonitrile, under the protection of argon, at 50 o C in a constant temperature oil bath for 24 h. After the reaction, the solvent was distilled off under reduced pressure and separated by column chromatography. The eluent containing the target compound was collected with the DCM / methanol mixture as the eluent. After removing the solvent, the quaternary ammonium salt protected by tert-butyl group was obtained. product. Further, the tert-butyl protected quaternary ammonium salt product (1.0 eq.), triethylsilane (2.5 eq.) was dissolved in anhydrous DCM, and trifluoroacetic acid (13 eq.) was added dropwise under ice bath conditions, After returning to room temperature, th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com