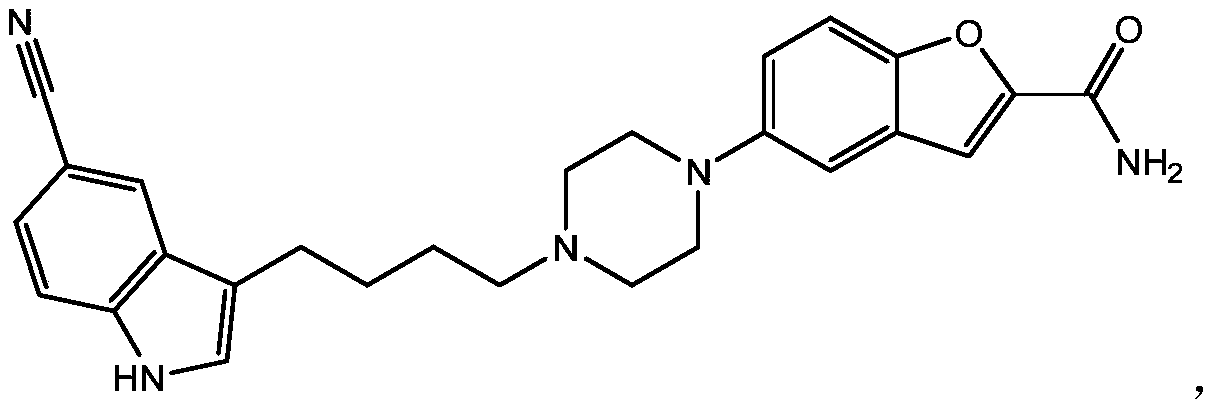
Vilazodone inclusion complexes, compositions and preparation thereof
A technology of composition and clathrate, applied in the direction of drug combination, non-active ingredients of polymer compounds, pharmaceutical formulations, etc., can solve the problem of low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0090] Those skilled in the art will appreciate that the following examples are intended to illustrate the invention and are not to be construed as limiting the invention. Various modifications and variations of the present invention will occur to those skilled in the art. Unless otherwise specified, if no specific technique or condition is explicitly described in the following embodiments, those skilled in the art can perform the procedure according to the commonly used technique or condition in the field or according to the product specification. The medicines, reagents or instruments used were not indicated by the manufacturer, and they were all commercially available conventional products.
[0091] Among them, the reference preparation Vilazodone hydrochloride tablets with a specification of 10 mg / tablet developed for Merck in Germany.
Embodiment approach
[0092] Unless otherwise specified, the following embodiments adopt the following detection methods:
[0093] HPLC instrument model: Agilent 1260
[0094] Chromatographic conditions: detection wavelength: UV242nm, chromatographic column: kromasil 100-5C18 4.6mm×150mm, 5 microns, mobile phase: 0.02M pH6.0 dipotassium hydrogen phosphate and acetonitrile are 54:46 (V / V, volume ratio), Flow rate: 1.0mL / min, injection volume: 10 microliters, running time: 4.5min.
Embodiment 1
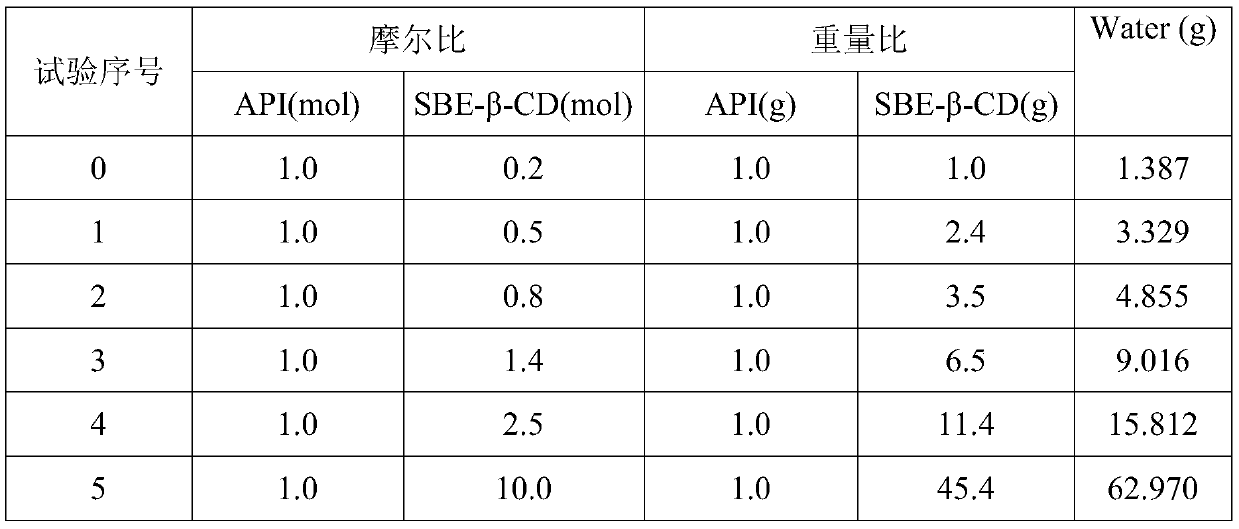
[0109] In Example 1, vilazodone hydrochloride (API) and sulfobutyl-β-cyclodextrin (SBE-β-CD) were stirred in a water bath at 80°C for 4 hours according to the prescription ratio in Table 4 to obtain the clathrate solution, and then freeze-dried to obtain a powder clathrate composition.
[0110] Table 4 The prescription ratio of vilazodone hydrochloride and sulfobutyl-β-cyclodextrin
[0111]
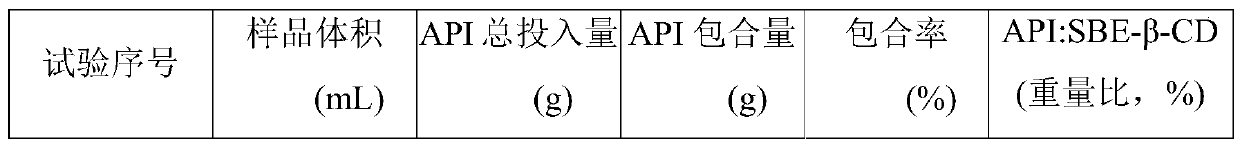
[0112] Each sample of the clathrate solution was filtered through a 0.45 micron filter membrane, and the filtrate was taken for HPLC testing to determine the content of the drug in the clathrate solution. Inclusion rate=included drug amount / total drug amount×100%. The results are shown in Table 5.
[0113] Table 5 drug inclusion amount and inclusion rate
[0114]
[0115]
[0116] According to the method in Comparative Example 1, in 0.1N HCl and pH 6.8 buffer solution, the vilazodone inclusion complex 1-0 to 1-5, C1 and the reference preparation (RLD) (each 10mg) were carried ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com