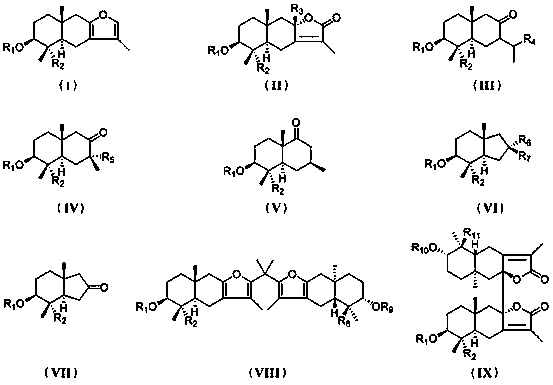
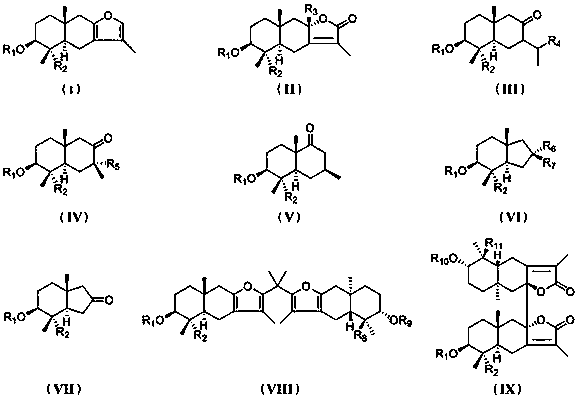
Eucalyptus sesquiterpene compounds as well as pharmaceutical composition, preparation method and application thereof
A technology of compounds, drugs, applied in the field of natural medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0110] Extraction, separation and purification of compound 1-68 of the present invention:
[0111] Dry the rhizomes (19 kg) of the genus Chiapodium in the shade, crush them to 30 meshes, extract them three times with 95% methanol at room temperature, 70 L each time for 24 hours, combine the extracts, and concentrate the extracts under reduced pressure to obtain Suspend the extract with an appropriate amount of water, and distribute it several times with ethyl acetate to obtain an ethyl acetate extract (1.5 kg). The extract is dissolved in an appropriate amount of chloroform / acetone and mixed with silica gel 80-100 mesh, and then mixed with 650 G silica gel 200-300 mesh was used for column chromatography, and the gradient elution was carried out with chloroform / acetone (1 : 0-0 : 1), and 8 main parts were obtained. The pure chloroform part, 9: 1 chlorine / acetone Silica gel column chromatography, 8 : 2 chlorine / C fraction and 7 : 3 chlorine / C fraction were carried out by gra...
Embodiment 2
[0113] Physical and spectral data of compounds 1, 9, 22, 30, 36, 43, 48, 54, 61 of the present invention:
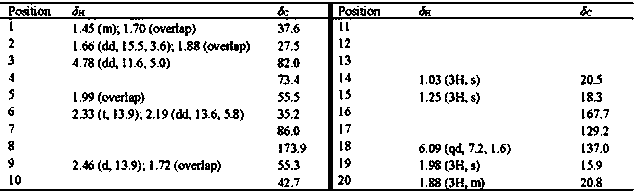
[0114] Compound 1: Pale yellow gum. HR-ESI-MS m / z : 331.1917 [M-H] - (calcd. for C 20 h 27 o 5 331.1915); [α] D 25 0 ( c = 0.085, MeOH); 1 H-NMR (400 MHz, CD 3 COCD 3 ) δ : 6.97 (1H, s,H-12), 5.95 (1H, qd, J = 7.2, 1.4 Hz, H-3'), 4.64 (1H, dd, J = 11.4, 4.2 Hz,H-3), 2.59 (1H, dd, J = 15.8, 4.7 Hz, H-6), 2.23 (2H, q, J = 15.4 Hz, H-9), 2.12 (1H, overlap, H-6), 1.85 (3H, dd, J = 7.2, 1.6 Hz, H-4'), 1.79 (3H, d, J = 1.3 Hz, H-13), 1.76 (3H, t, J = 1.6 Hz, H-5'), 1.71 (1H, m, H-2), 1.62 (1H,dd, J = 11.7, 4.5 Hz, H-5), 1.56 (1H, overlap, H-1), 1.50 (1H, overlap, H-2),1.46 (1H, overlap, H-1), 1.20 (3H, s, H-15), 0.80 (3H, s, H-14); 13 C-NMR (100MHz, CD 3 COCD 3 ) δ : 39.2 (t, C-1), 26.2 (t, C-2), 81.7 (d, C-3), 74.1 (s, C-4), 51.0 (d, C-5), 18.1 (t, C-6), 117.1 (s, C-7), 149.7 (s, C-8), 42.7 (t, C-9), 35.7 (s, C-10), 120.2 (s, C-11), 138.0...
Embodiment 3
[0145] Antimalarial activity detection of compounds of the present invention:
[0146] Using the internationally accepted 4-day inhibition test method, each mouse was inoculated intraperitoneally with 1×10 7 Plasmodium-infected red blood cells were given intragastric administration 3 hours after inoculation with Plasmodium, and then administered once every 24 hours for 4 consecutive days (the inoculation day was D 0 , the next day is D 1 , and so on), on day 5 (D 4 ) blood from the tail vein, coated with a thin blood film, fixed in methanol, stained with Wright-Giemsa mixed staining method, and observed under a 10×100 oil microscope. If no Plasmodium asexual body is found after randomly checking 50 fields of view, it is judged as negative, and the positive thin blood film is randomly counted in 5 fields of view, and the total number of red blood cells is not less than 1000, and then the inhibition rate of Plasmodium is calculated according to the following formula .
[014...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com