A New Method for Determining the Integrity of Type I Collagen Triple Helix Structure
A technology for structural integrity and collagen, which is applied in the field of judging the integrity of type I collagen triple helix, can solve the problems of collagen triple helix structure damage and affect biological activity, etc., to achieve quantitative analysis, good reproducibility, Simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] (1) Take the collagen sample of aquatic carp skin to be tested, add pre-cooled 0.5M acetic acid solution, dissolve it at 10°C and divide it into tubes A and B, and add an appropriate amount of pepsin;
[0073] (2) Tube A is always in a water bath at 10°C, and tube B is placed in a water bath at 40°C;
[0074] (3) At the set time, draw samples from tubes A and B into tubes A1 and B1 respectively;
[0075] (4) Place tubes A1 and B1 in an ice water bath quickly, and add pepsin inhibitor (Pepstatin);
[0076] (5) Repeat the operation from steps (3) to (4) and change the set time. A1 and B1 are obtained at 0 minutes, A2 and B2 at 30 minutes, A3 and B3 at 1 hour, and A4 and B4 at 2 hours , get A5, B5 at 3h, A6, B6 at 5h;
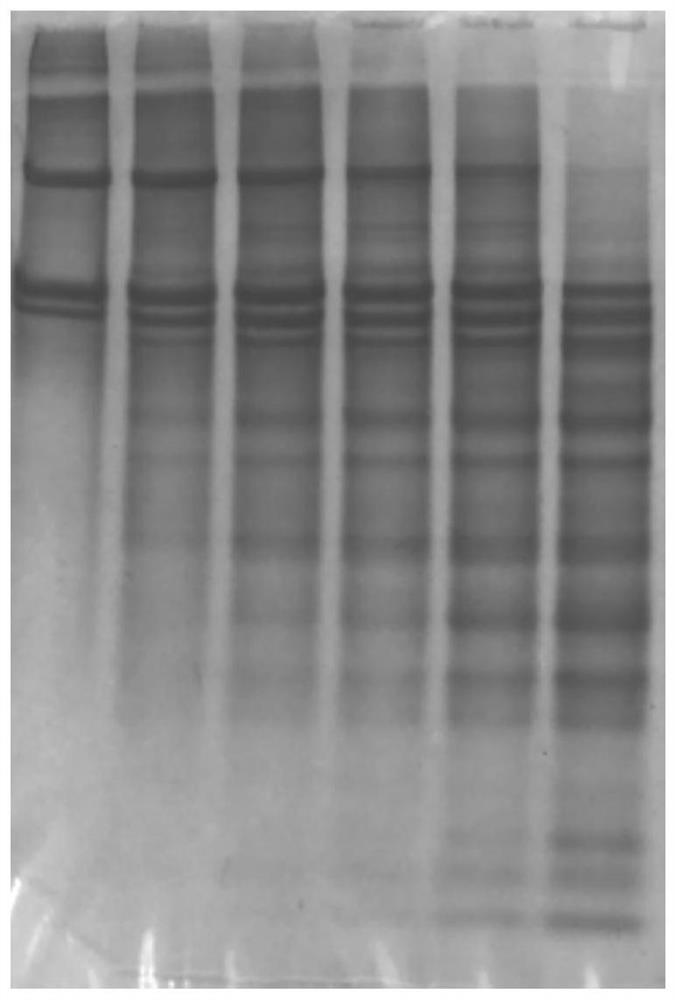
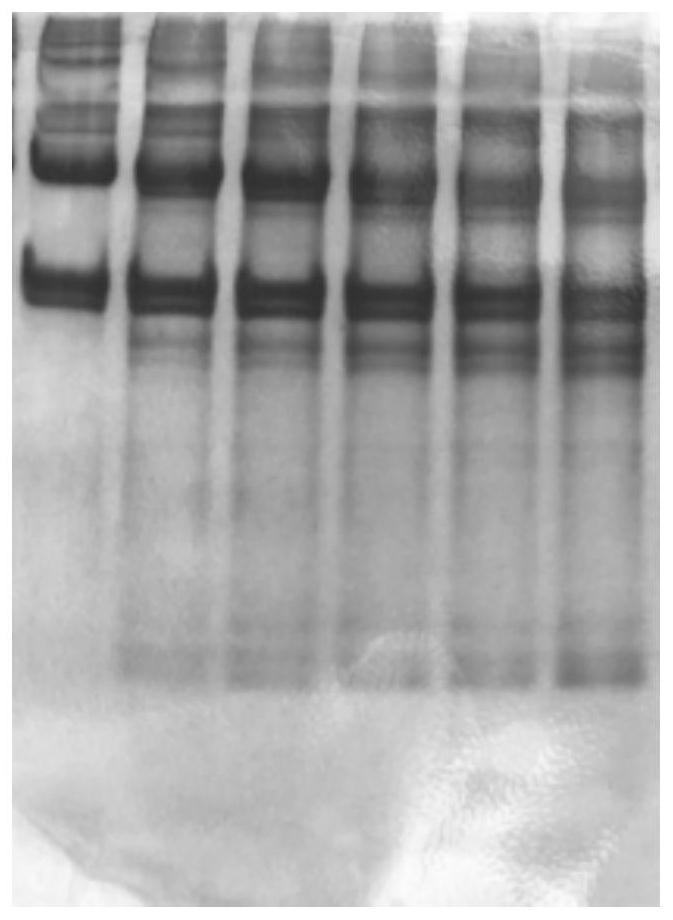
[0077] (6) Prepare electrophoresis samples from the obtained A1-6 and B1-6 samples, such as figure 1 and 2 as shown, figure 1 The 6 bands shown in are respectively A1, A2, A3, A4, A5, A6, figure 2 The six bands shown in are B1, B2, B3, B4, B5, and B6...
Embodiment 2
[0083] (1) Take the collagen-rich animal tissue pigskin collagen sample to be tested, add pre-cooled 0.1M acetic acid solution, dissolve at 5°C and divide into tubes A and B, and add appropriate amount of pepsin;
[0084] (2) Tube A is always in a water bath at 0°C, and tube B is placed in a water bath at 80°C;
[0085] (3) At the set time, draw samples from tubes A and B into tubes A1 and B1 respectively;
[0086](4) Place tubes A1 and B1 in an ice water bath quickly, and add pepsin inhibitor (Pepstatin);
[0087] (5) Repeat the operation from steps (3) to (4) and change the set time. A1 and B1 are obtained at 0 minutes, A2 and B2 at 30 minutes, A3 and B3 at 1 hour, and A4 and B4 at 2 hours , get A5, B5 at 3h, A6, B6 at 5h;
[0088] (6) Prepare electrophoresis samples from the obtained A1-6 and B1-6 samples; image 3 and 4 as shown, image 3 The 6 bands shown in are respectively A1, A2, A3, A4, A5, A6, Figure 4 The 6 bands shown in are B1, B2, B3, B4, B5, B6 respective...
Embodiment 3
[0094] (1) Take the beef tendon collagen sample of animal tissue rich in collagen to be tested, add pre-cooled 0.5M acetic acid solution, dissolve at 0°C, and divide into tubes A and B, and add appropriate amount of pepsin;
[0095] (2) Tube A is always in a water bath at 10°C, and tube B is placed in a water bath at 70°C;
[0096] (3) At the set time, draw samples from tubes A and B into tubes A1 and B1 respectively;
[0097] (4) Place tubes A1 and B1 in an ice water bath quickly, and add pepsin inhibitor (Pepstatin);
[0098] (5) Repeat the operation from steps (3) to (4) and change the set time. A1 and B1 are obtained at 0 minutes, A2 and B2 at 30 minutes, A3 and B3 at 1 hour, and A4 and B4 at 2 hours , get A5, B5 at 3h, A6, B6 at 5h;
[0099] (6) Prepare electrophoresis samples from the obtained A1-6 and B1-6 samples; Figure 5 and 6 as shown, image 3 The 6 bands shown in are respectively A1, A2, A3, A4, A5, A6, Figure 4 The 6 bands shown in are B1, B2, B3, B4, B5,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com