A kind of zopiclone artificial hapten, artificial antigen and preparation method and application thereof
An artificial hapten, zopiclone technology, applied in the direction of animal/human protein, organic chemistry, specific peptides, etc., can solve the problem of artificial hapten and artificial antigen without zopiclone, and achieve high sensitivity and specificity. , the effect of high immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
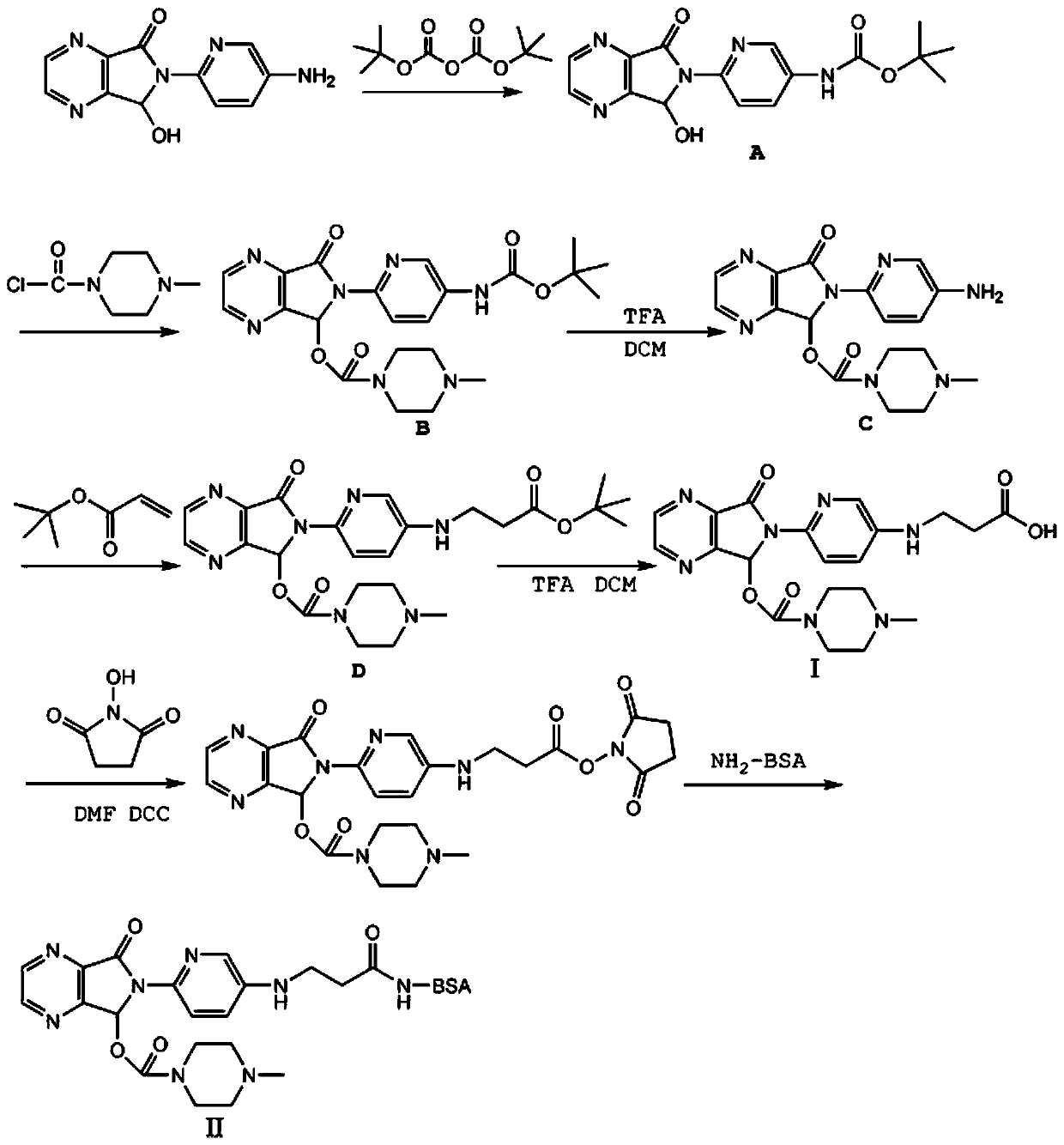
[0055] The preparation method of this implementation a kind of zopiclone artificial antigen (reaction process is as follows figure 1 ), including the following steps:
[0056] (1) Preparation of artificial hapten:
[0057] ① Weigh 500 mg (2.06 mmol) of 6-(5-amino-2-pyridyl)-6,7-dihydro-7-hydroxyl-5H-pyrrolo[3,4-b]pyrazin-5-one In a 50ml round bottom flask, add 20ml tetrahydrofuran and 5ml water to dissolve, then add 519μL (2.26mmol) of di-tert-butyl dicarbonate and 240mg (2.26mmol) of sodium carbonate, put it into a stirring bar, stir the reaction at room temperature for 3 hours, and the reaction is over , the solvent was evaporated to dryness under reduced pressure, 30ml of purified water was added, pH=3 was adjusted with 1N hydrochloric acid, and extracted twice with 30ml of ethyl acetate, the organic phase was collected, dried with anhydrous magnesium sulfate, filtered, and evaporated to dryness under reduced pressure to obtain Pale yellow oily product A548mg (1.60mmol); ...
Embodiment 2
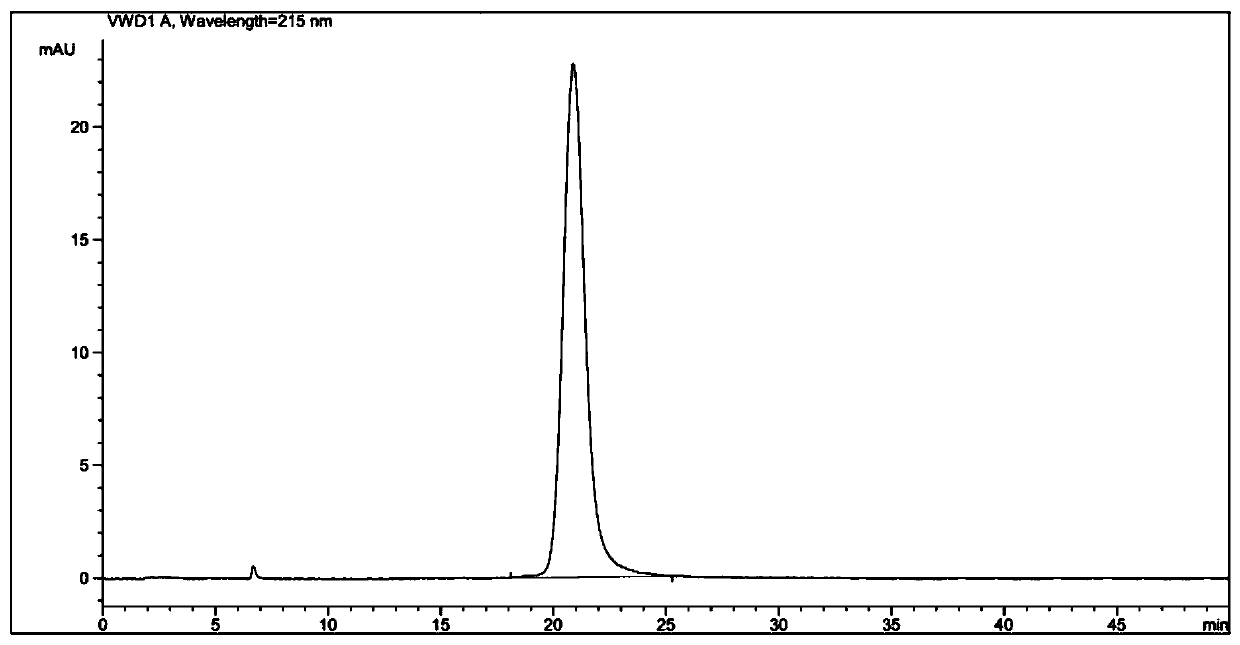
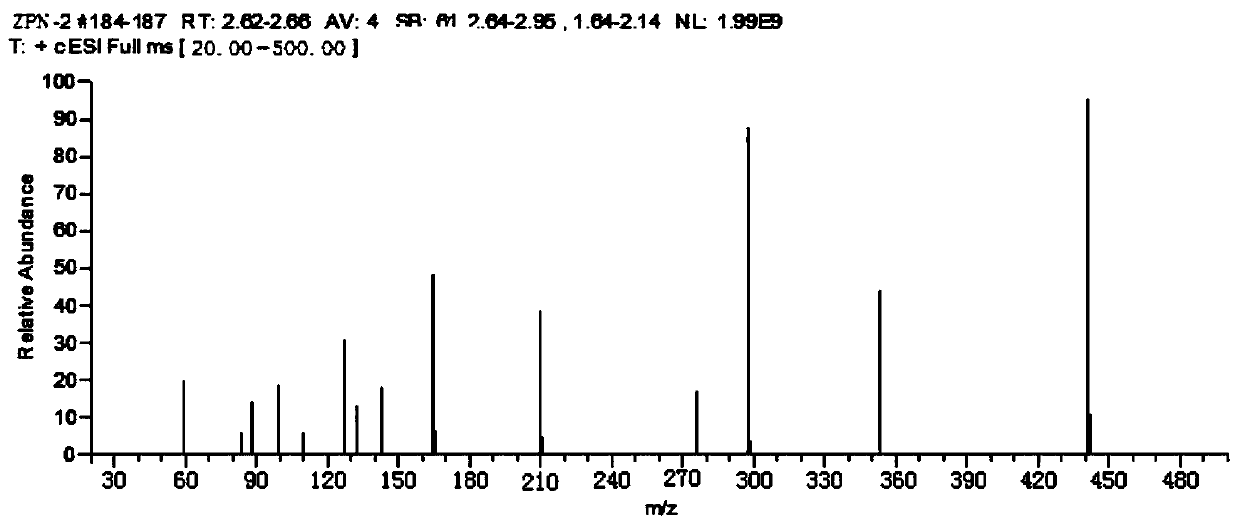
[0138] The performance measurement of embodiment 2 zopiclone artificial antigen
[0139] (1) Identification of zopiclone artificial antigen:
[0140] Molar absorption coefficient ε: Zopiclone artificial hapten solutions with concentrations of 0 μg / ml, 5 μg / ml, 10 μg / ml, 20 μg / ml, 30 μg / ml, and 40 μg / ml were prepared with PBS buffer solution, which can be seen from the ultraviolet scanning diagram The maximum absorption wavelength of the zopiclone hapten is 273nm, the absorbance value is measured at 273nm, and parallel samples are made for each concentration. The formula for calculating the molar absorptivity (ie, the molar absorptivity) is: ε=absorbance value / molar concentration.
[0141]Determination of conjugate protein concentration: Prepare the concentration of 0μg / ml, 10μg / ml, 20μg / ml, 30μg / ml, 40μg / ml, 60μg / ml, 80μg / ml, 100μg / ml, 120μg / ml in PBS buffer Add 3ml of Coomassie Brilliant Blue staining solution to 1ml of bovine serum albumin solution, mix immediately, warm i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com