A kind of ethyl sulfate artificial antigen, preparation method and application
A technology of ethyl sulfate and artificial antigen, which is applied in the direction of preparation of sulfate ester, preparation method of peptide, chemical instrument and method, etc., can solve the problem of poor mastery, affecting the accuracy of detection and inspection results, and affecting the accuracy of alcohol inspection results, etc. problems, to achieve the effect of few influencing factors, high accuracy and accurate detection results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
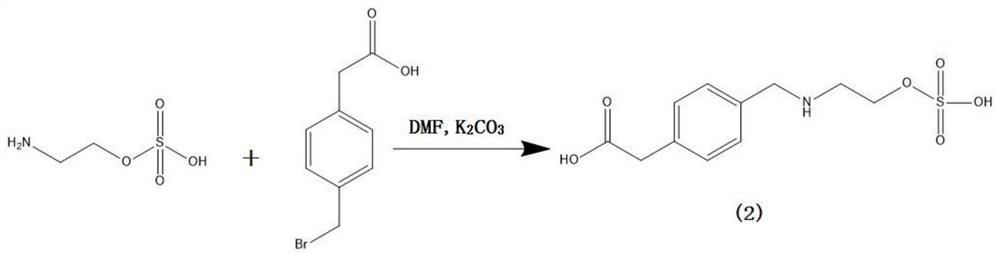
[0036] (1) Preparation of ethyl sulfate hapten
[0037] Weigh 100mg (0.71mmol) of 2-aminoethanol hydrogen sulfate, add it to a 50ml single-necked round bottom flask, then add 5ml of N,N-dimethylformamide (DMF), 276mg (2mmol) of potassium carbonate and 300mg (1.31 mmol) 2-(4-(bromomethyl)phenyl)acetic acid, a stirring bar was added, and the reaction was stirred at room temperature for 20 hours; after the reaction was completed, the solvent was evaporated to dryness under reduced pressure, and purified by thin-layer chromatography, TLC: chromatographic solution It is dichloromethane:methanol=1:1 (volume ratio), product ratio shift value Rf=0.3~0.4; 86mg of hapten (2) is obtained, and the specific synthetic route is as follows figure 1 shown.
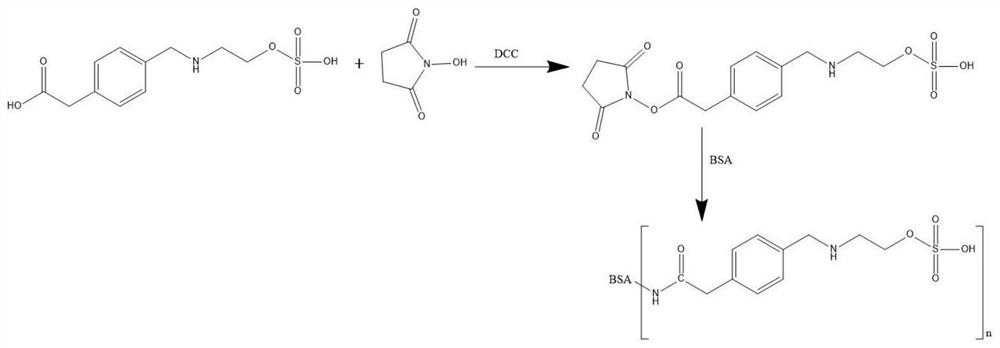
[0038] (2) Preparation of ethyl sulfate artificial antigen
[0039] a. Weigh 50mg (0.17mmol) of ethyl sulfate hapten into a 50ml round-bottomed flask, add 2.5ml of N,N-dimethylformamide (DMF), and then add 39mg (0.34mmol) of N-hydroxysuc...
Embodiment 2
[0053] (1) Preparation of ethyl sulfate hapten
[0054] Weigh 100mg (0.71mmol) of 2-aminoethanol hydrogen sulfate, add it to a 50ml one-neck round bottom flask, then add 5ml of N,N-dimethylformamide (DMF), 138mg (1mmol) of potassium carbonate and 200mg (0.87 mmol) 2-(4-(bromomethyl)phenyl)acetic acid, a stirring bar was added, and the reaction was stirred at room temperature for 20 hours; after the reaction was completed, the solvent was evaporated to dryness under reduced pressure, and purified by thin-layer chromatography, TLC: chromatographic solution It is dichloromethane: methanol=1:1 (volume ratio), the product ratio shift value Rf=0.3~0.4; obtain hapten (2) 40mg, the specific synthetic route is as follows figure 1 shown.
[0055] (2) Preparation of ethyl sulfate artificial antigen
[0056] a. Weigh 40mg (0.14mmol) of ethyl sulfate hapten into a 50ml round-bottomed flask, add 2ml of N,N-dimethylformamide (DMF), and then add 24mg (0.21mmol) of N-hydroxysuccinimide Amin...
Embodiment 3
[0063] (1) Preparation of ethyl sulfate hapten
[0064] Weigh 100mg (0.71mmol) of 2-aminoethanol hydrogen sulfate, add it to a 100ml single-neck round bottom flask, then add 10ml of N,N-dimethylformamide (DMF), 552mg (4mmol) of potassium carbonate and 600mg (2.62 mmol) 2-(4-(bromomethyl)phenyl)acetic acid, a stirring bar was added, and the reaction was stirred at room temperature for 20 hours; after the reaction was completed, the solvent was evaporated to dryness under reduced pressure, and purified by thin-layer chromatography, TLC: chromatographic solution It is dichloromethane: methanol=1:1 (volume ratio), the product ratio shift value Rf=0.3~0.4; obtain the hapten (2), the specific synthetic route is as follows figure 1 shown.
[0065] (2) Preparation of ethyl sulfate artificial antigen
[0066] a. Weigh 50mg (0.17mmol) of ethyl sulfate hapten into a 100ml round-bottomed flask, add 10ml of N,N-dimethylformamide (DMF), and then add 1.1mmol of N-hydroxysuccinimide (NHS) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com