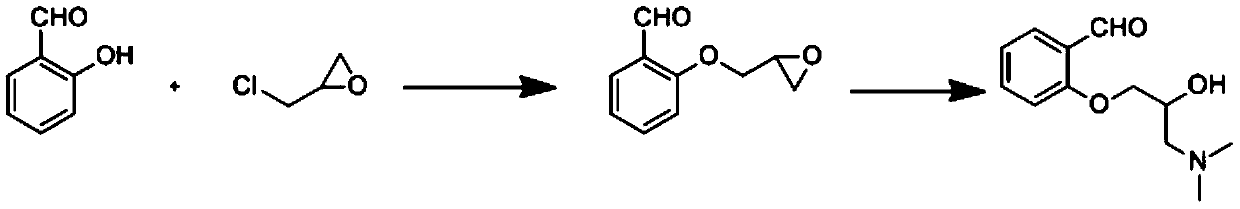
Method for synthesizing 2-(3-dimethylamino-2-hydroxy) propoxybenzaldehyde as intermediate of sarpogrelate hydrochloride
A technology of sagrelate hydrochloride and propoxybenzaldehyde, which is applied in the field of synthesis of 2-propoxybenzaldehyde, an intermediate of sarcogrelate hydrochloride, can solve problems such as difficulty in control, increased cost, cumbersome process, etc., and prevent palladium Effects of carbon catalyst poisoning, guaranteed recycling, and simplified synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Put 150g epichlorohydrin into 100g salicylaldehyde, stir, put 120g anhydrous potassium carbonate under nitrogen protection, heat up to 80±2°C, react for 5h, recover epichlorohydrin under reduced pressure, pour the residual liquid into 300mL ice water, The organic layer was separated to obtain 143g of intermediate with a yield of 98% and a purity of 96%.
[0024] Under the protection of nitrogen, put 143g of the intermediate into 429g of 30% dimethylamine aqueous solution, stir and react at 25±2°C for 12h, after the reaction is completed, adjust the pH to 7 with dilute hydrochloric acid, leave to separate and separate to obtain an oil layer, wash twice with water, and anhydrous After drying over magnesium sulfate, a yellow-brown oily liquid was obtained, and 155 g of 2-(3-dimethylamino-2-hydroxy)propoxybenzaldehyde was obtained with a purity of 92.3% and a yield of 86%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com