Methods for synthesizing natural and non-natural protopanaxatriol ginsenosides
A technology of protopanaxatriol and ginsenoside, which is applied in the fields of biotechnology and botany, can solve the problems of poor stereoselectivity, many side reactions, and large pollution in chemical methods, and achieves the effect of enriching varieties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
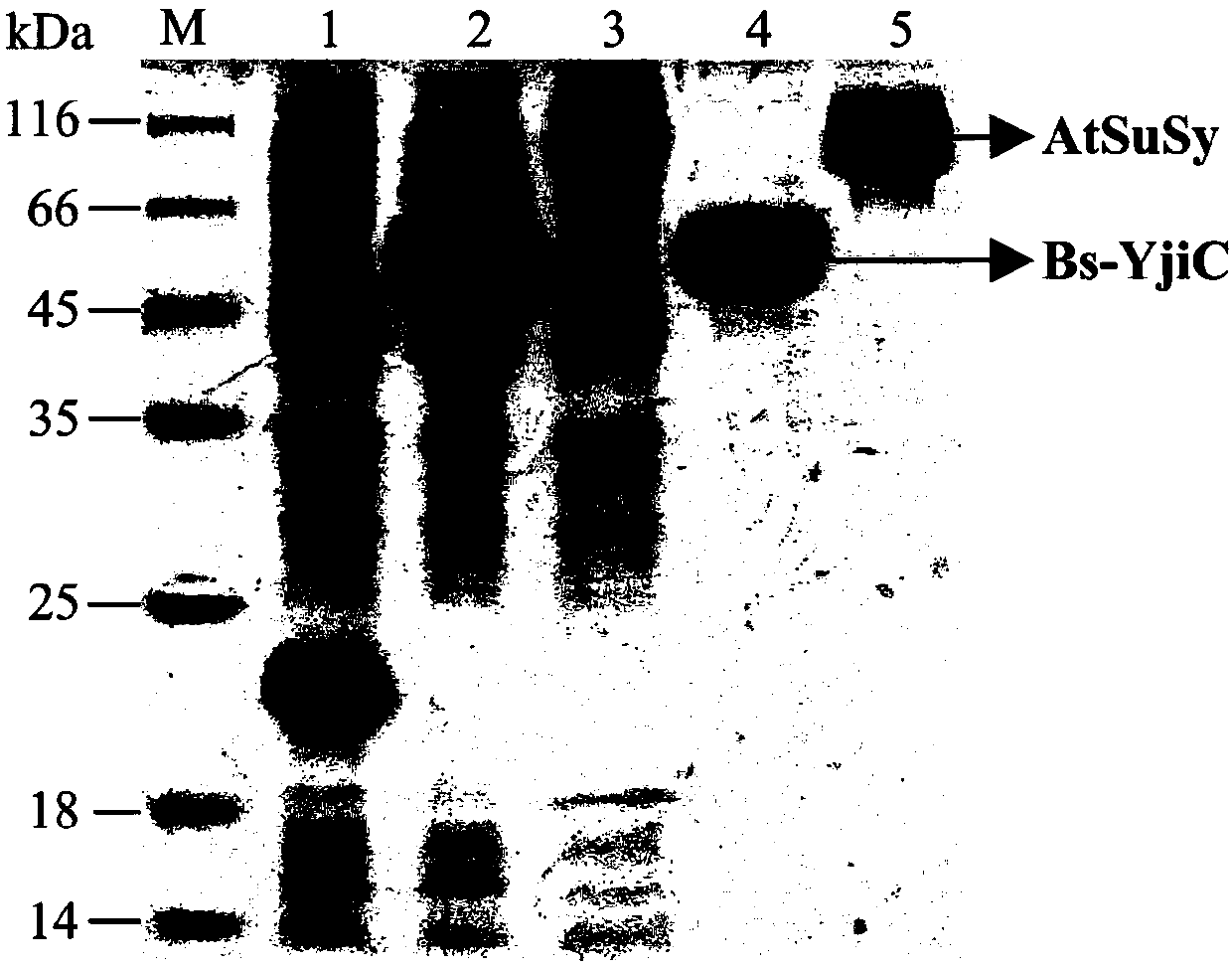
[0056] Example 1. Cloning, expression and purification of glycosyltransferase Bs-YjiC and sucrose synthase AtSuSy
[0057] The gene accession numbers of glycosyltransferase Bs-YjiC and sucrose synthase AtSuSy in NCBI (https: / / www.ncbi.nlm.nih.gov / ) are NP_389104 and NM_001036838, respectively, and the genomic DNA of Bacillus subtilis 168 As template, use Bs-YjiC-F and Bs-YjiC-R primer pair to amplify glycosyltransferase Bs-YjiC gene; use Arabidopsis cDNA as template, use At-SuSy-F and AtSuSy-R primer pair to amplify Increased sucrose synthase AtSuSy. The nucleotide sequences of the primers used are as follows (the underlined part is the restriction enzyme site):
[0058] Bs-YjiC-F: 5′-CGCGGATCCATGAAAAAGTACCATATTTCGAT-3′ (BamHI restriction site)
[0059] Bs-YjiC-R: 5′-CGCGTCGACTTACTGCGGGACAGCGGATTTT-3′ (SalI restriction site)
[0060]AtSuSy-F: 5′-GCGTCGACAAATGGCAAACGCTGAACGTATGATAA-3′ (SalI restriction site)
[0061] AtSuSy-R: 5′-TTGCGGCCGCTTATCATACGTTCAGCGTTTGCCAT-3′(NotI ...
Embodiment 2
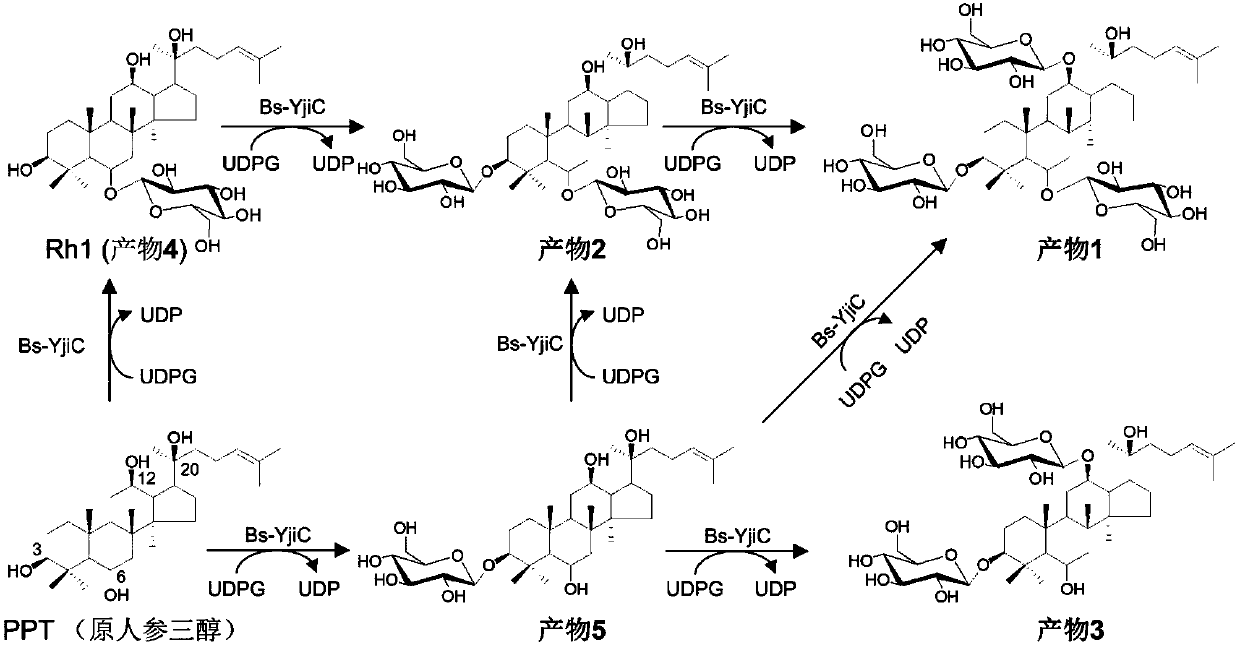
[0063] Example 2. Glycosyltransferase Bs-YjiC catalyzes glycosylation of protopanaxatriol
[0064] Separation and purification of glycosyltransferase Bs-YjiC according to the steps described in Example 1. The reaction system for glycosylation of protopanaxatriol catalyzed by glycosyltransferase Bs-YjiC includes: 2mM protopanaxatriol, 10mM uridine diphosphate glucose, 50mM Tris-HCl (pH 7.5), 10mM MgCl 2 , and 5mU / mL glycosyltransferase Bs-YjiC, reacted at 35°C for 0.5h.
[0065] After the reaction is completed, add an equal volume of methanol to terminate the reaction, then centrifuge at 12,000 rpm for 10 minutes, take the supernatant and filter it through a 0.22 μm filter membrane, add it to a liquid phase bottle, and pass through high performance liquid chromatography and high performance liquid chromatography-mass spectrometry Analysis and identification of glycosylation products. The analysis conditions of high-performance liquid chromatography are: mobile phase A is dist...
Embodiment 3
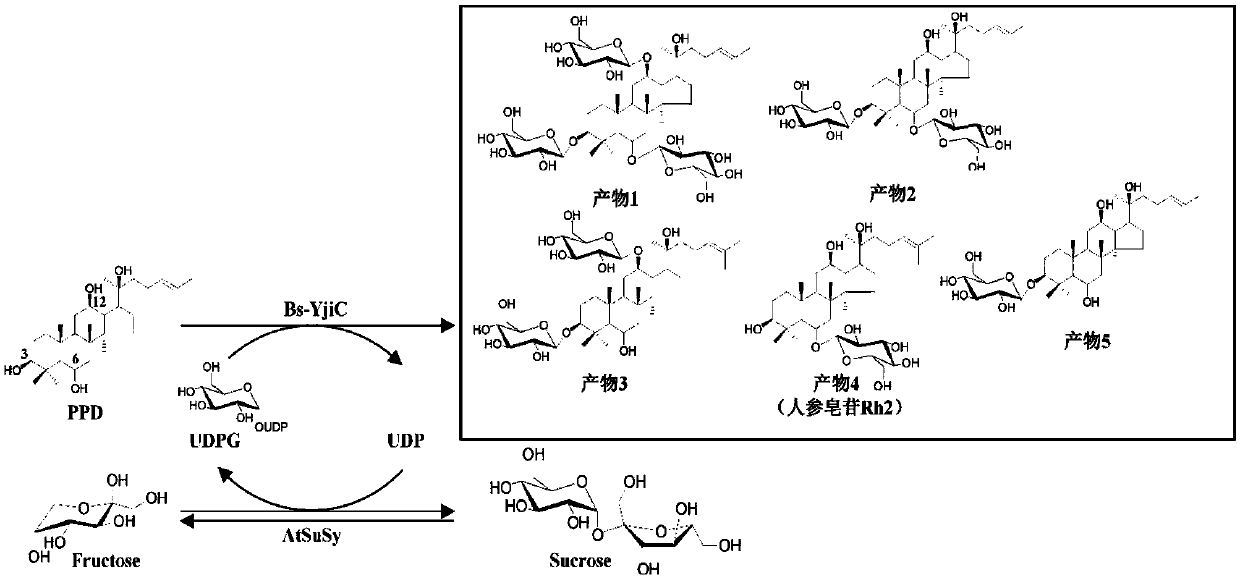
[0068] Example 3. Coupling reaction between glycosyltransferase Bs-YjiC and sucrose synthase AtSuSy catalyzes glycosylation of protopanaxatriol
[0069] The coupling reaction of glycosyltransferase Bs-YjiC and sucrose synthase AtSuSy to catalyze the glycosylation of protopanaxatriol is as follows: 3mM protopanaxatriol, 0.5mM uridine diphosphate, 50mM Tris-HCl (pH7.5) , 10 mM MgCl 2 , 10% dimethyl sulfoxide (v / v), 160mU / mL glycosyltransferase Bs-YjiC, 200mU / mL sucrose synthase AtSuSy, react at 35°C, and react for 1h at 200rpm. After the reaction, the reaction product was identified according to the reaction conditions in Example 2 and the detection method of high performance liquid chromatography. Such as Figure 5 It shows that, like the reaction system using expensive uridine diphosphate glucose as the glycosyl donor, the double-enzyme coupling reaction system composed of glycosyltransferase Bs-YjiC and sucrose synthase AtSuSy can use cheap sucrose as the glycosyl donor. D...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com