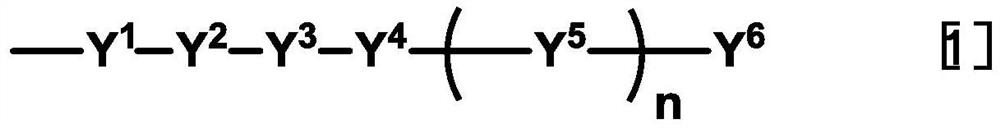Liquid crystal display element with curved liquid crystal panel and liquid crystal aligning agent used therefor
A technology of liquid crystal aligning agent and liquid crystal panel, which is applied in the directions of instruments, optics, nonlinear optics, etc., and can solve the problem of not being able to limit the orientation of liquid crystals.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0165] Although an Example is given below and this invention is demonstrated more concretely, it is not limited to these. It should be noted that, in the following, the abbreviations used are as follows.
[0166] (tetracarboxylic dianhydride)
[0167] CBDA: 1,2,3,4-cyclobutanetetracarboxylic dianhydride
[0168] BODA: Bicyclo[3,3,0]octane-2,4,6,8-tetracarboxylic dianhydride
[0169]
[0170] 3-AMPDA: 3,5-diamino-N-(pyridin-3-ylmethyl)benzamide
[0171] p-PDA: p-phenylenediamine
[0172] PBCH5DAB: 1,3-Diamino-4-{4-[trans-4-(trans-4-n-pentylcyclohexyl)cyclohexyl]phenoxy}benzene
[0173] PCH7DAB: 1,3-Diamino-4-[4-(trans-4-n-heptylcyclohexyl)phenoxy]benzene
[0174]
[0175] TEOS: Tetraethoxysilane
[0176] UPS: 3-ureidopropyltriethoxysilane
[0177] MPMS: 3-Methacryloxypropyltrimethoxysilane
[0178] HTMS: Hexadecyltrimethoxysilane
[0179] (Organic solvents)
[0180] NMP: N-methyl-2-pyrrolidone, NEP: N-ethyl-2-pyrrolidone
[0181] BCS: Butyl cellosolve, PB: Prop...
Synthetic example 1
[0196] BODA (23.64g, 94.5mmol), p-PDA (5.11g, 47.3mmol), 3-AMPDA (16.0g, 66.15mmol) and DA-1 (32.86g, 75.6mmol) were mixed in NMP (216.0g) , After reacting at 80° C. for 5 hours, CBDA (18.53 g, 94.5 mmol) and NMP (94.6) g were added, and reacted at 40° C. for 6 hours to obtain a polyamic acid solution.
[0197] NMP (62.3g) was added to this polyamic acid solution (30.0g), and after diluting to 6% by mass, acetic anhydride (6.02g) and pyridine (1.87g) were added as an imidation catalyst, and the React for 3 hours. This reaction solution was poured into methanol (601.0 g), and the obtained deposit was collected by filtration. This deposit was wash|cleaned with methanol, it dried under reduced pressure at 100 degreeC, and the polyimide powder (A) was obtained. The imidization rate of this polyimide was 75%, Mn (number average molecular weight) was 13,200, and Mw (weight average molecular weight) was 40,600.
Synthetic example 2
[0199] BODA (23.64g, 94.5mmol), p-PDA (5.11g, 47.3mmol), 3-AMPDA (16.0g, 66.15mmol), DA-1 (16.43g, 37.8mmol) and DA-8 (14.39g, 37.8mmol) was mixed in NMP (207.8g), and after reacting at 80°C for 5 hours, CBDA (18.53g, 94.5mmol) and NMP (94.6)g were added, and reacted at 40°C for 6 hours to obtain a polyamic acid solution .
[0200]NMP (62.3 g) was added to this polyamic acid solution (30.0 g), and after diluting to 6% by mass, acetic anhydride (6.15 g) and pyridine (1.90 g) were added as an imidation catalyst, and the mixture was heated at 80° C. React for 3 hours. This reaction solution was poured into methanol (602.1 g), and the obtained deposit was collected by filtration. This deposit was wash|cleaned with methanol, it dried under reduced pressure at 100 degreeC, and the polyimide powder (B) was obtained. The imidization rate of this polyimide was 75%, Mn was 12,900, and Mw was 40,400.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com