Synthetic hydromagnesite particle and method for producing same
A technology of hydromagnesite and particles, applied in the field of synthetic hydromagnesite and its preparation, can solve the problems of long reaction time, irregular particle size of hydromagnesite, high reaction temperature, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] A 5-mol magnesium sulfate aqueous solution was prepared in a raw material tank with a capacity of 5 L. A 4-molar aqueous solution of sodium carbonate was additionally prepared, and poured into the above-mentioned aqueous solution of magnesium sulfate under stirring over about 15 minutes. An aqueous sodium hydroxide solution was dropped on the reactant to keep the pH of the reaction liquid at 10.89, and the reaction was carried out for 1 hour.
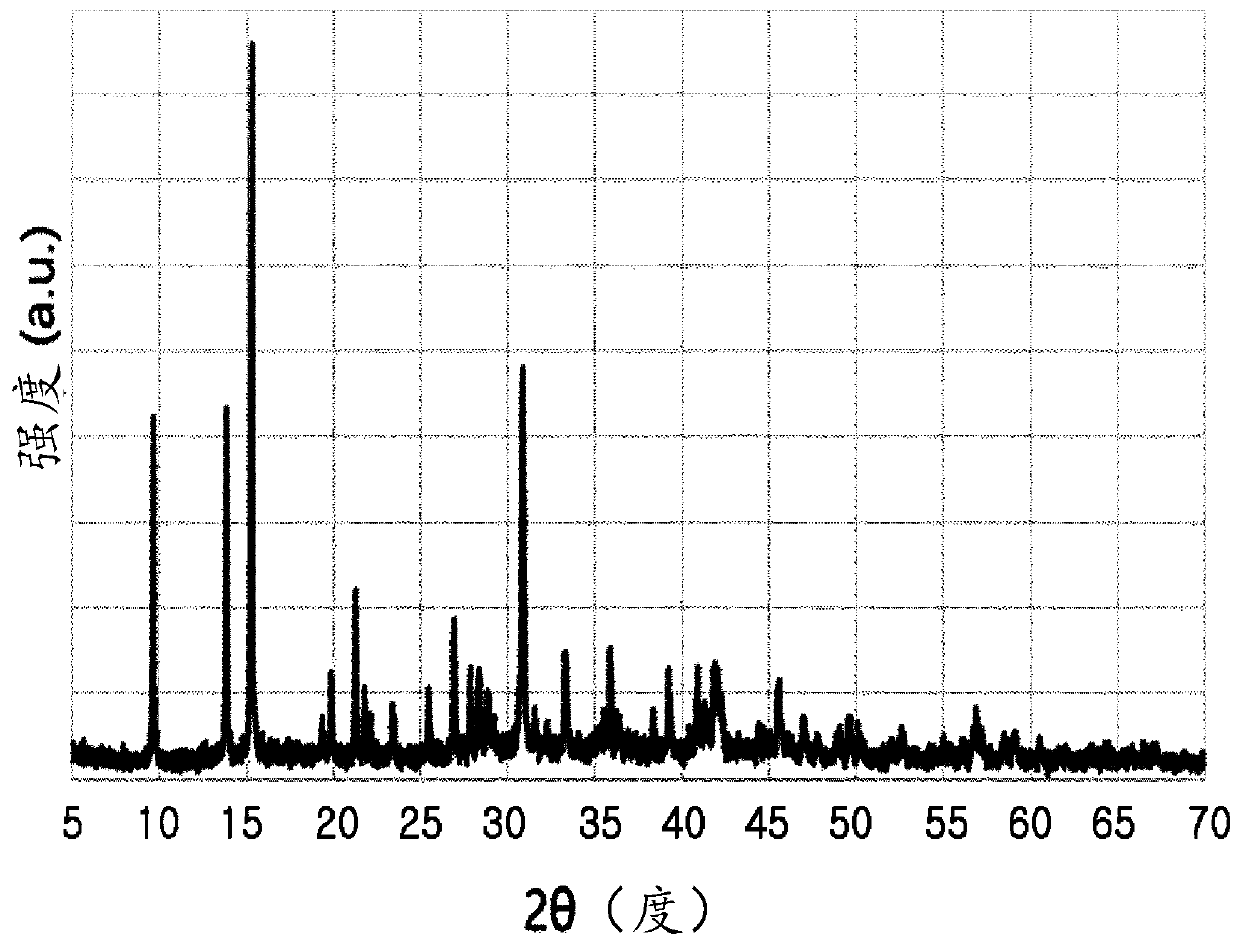
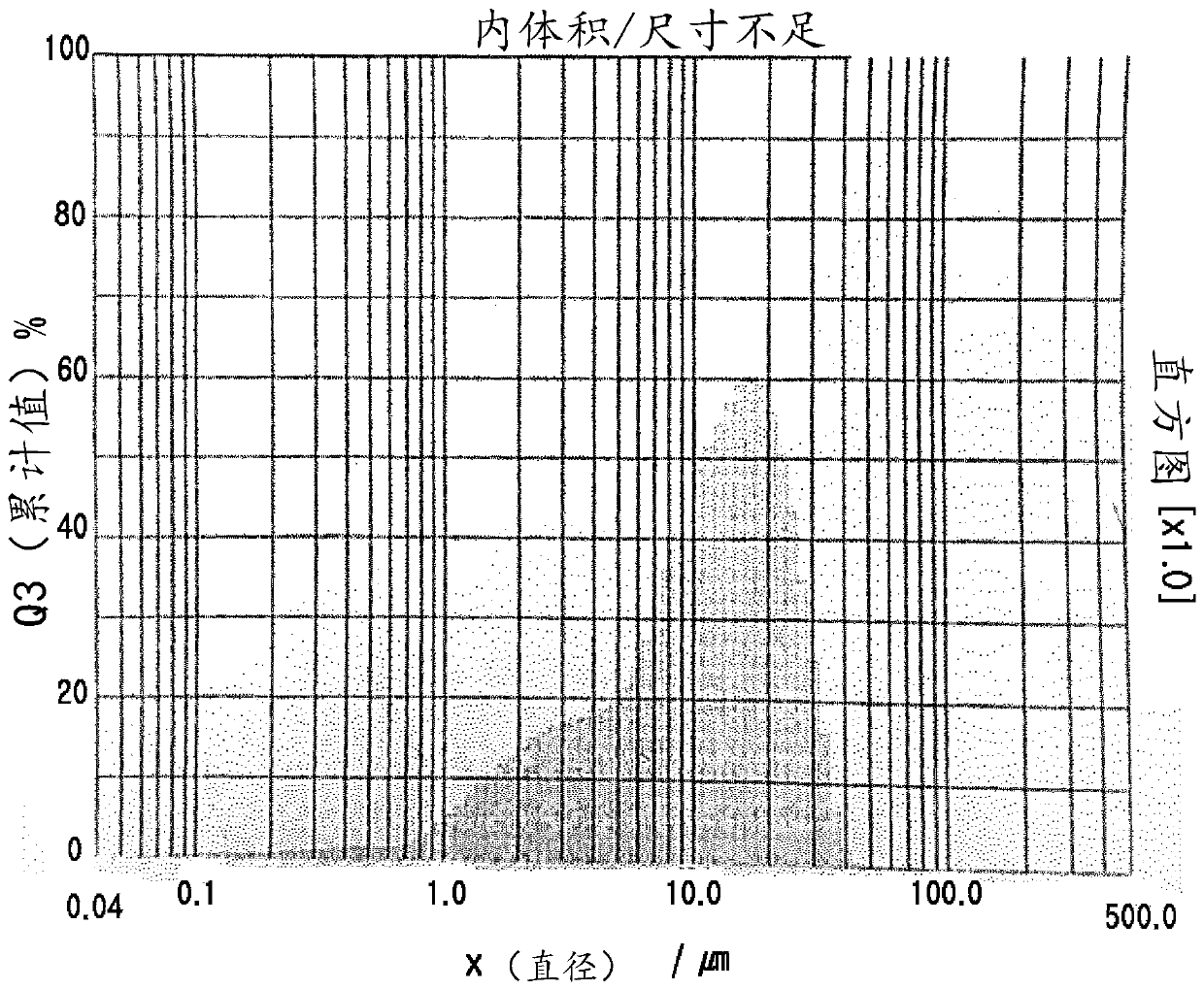
[0076] After the reaction, it was washed with water and dried with hot air at a temperature of 105° C. to obtain white powder synthetic hydromagnesite. Carry out elemental analysis, the result chemical formula is Mg 4.9 (OH) 2.05 (CO 3 ) 4.1 4H 2 O. and if Figure 5 to Figure 7 As shown, it was confirmed by electron microscope (scanning electron microscope) image, X-ray diffraction (XRD) image pattern and particle size distribution analysis. 2 / g.
Embodiment 2~ Embodiment 3 and comparative example 1~ comparative example 3
[0078] Synthetic hydromagnesite was obtained by reacting in the same manner as in Example 1 above with the reactants described in the table below, the pH of the reactants, and the time for feeding raw materials. Also, the particle size of the obtained synthetic hydromagnesite is described.
[0079] Table 1
[0080]
Example 2
Example 3
Comparative example 1
Comparative example 2
Comparative example 3
MgSO 4
5 moles
5 moles
5 moles
5 moles
5 moles
Na 2 CO 3
4 moles
4 moles
4 moles
4 moles
4 moles
pH value of reactant
10.54
10.28
9.12*)
9.61
11.20
temperature reflex
85℃
85℃
85℃
85℃
85℃
Raw material input time
15 minutes
15 minutes
15 minutes
15 minutes
15 minutes
Reaction time
1h
1h
1h
1h
1h
Particle size (μm)
1.87
1.49
13.07
5.72
4.34
[0081] *): Comparative example 1 is the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com