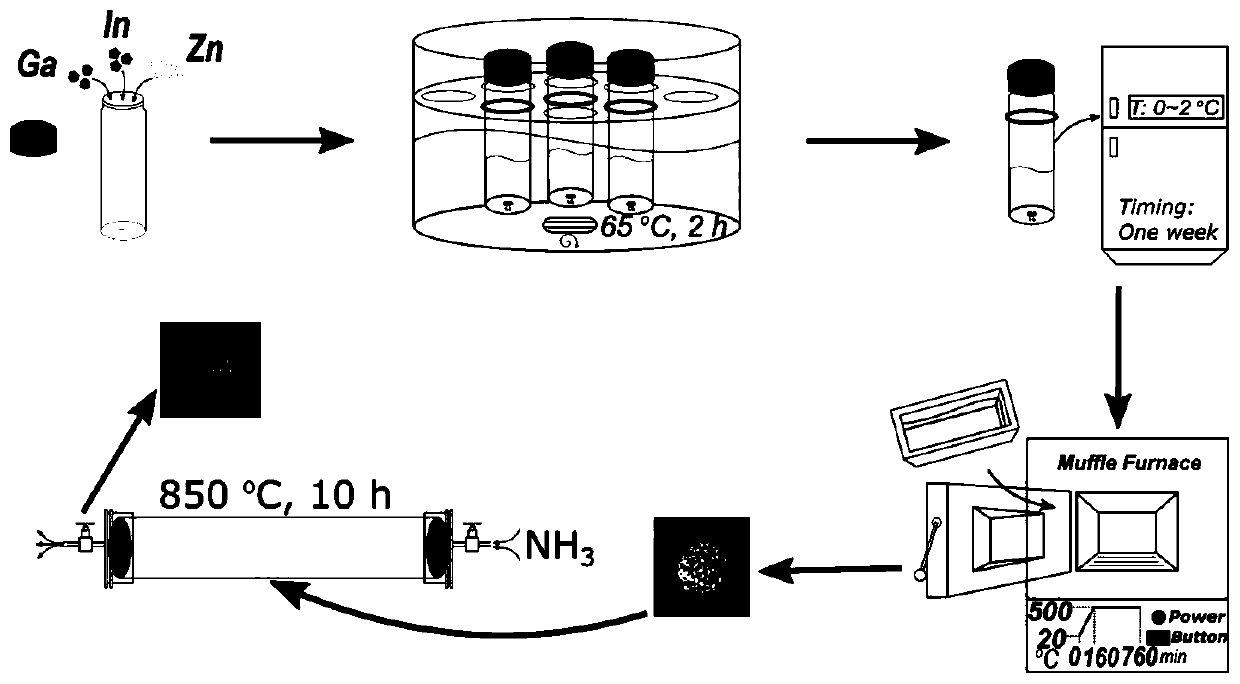
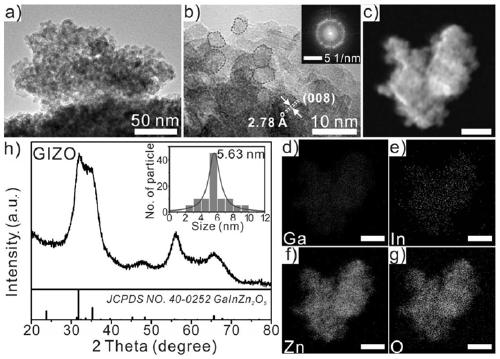
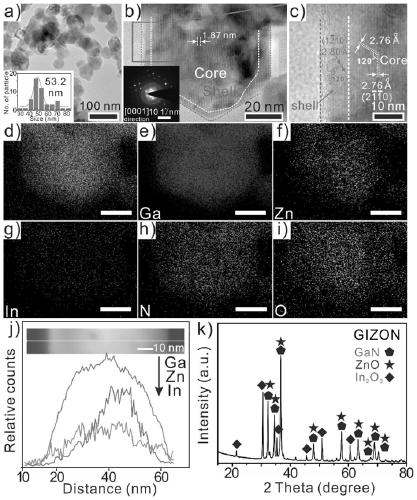
Preparation method and application of gallium-indium-zinc ternary nitrogen oxide
A nitrogen oxide, gallium indium zinc technology, applied in the field of chemistry, can solve the problems of less than 5% solar energy utilization rate, energy waste, affecting the catalytic effect, etc., to improve the photocatalytic complete water splitting activity, good crystallinity, and ensure uniformity. sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] In order to optimize the preparation process of this type of material, the present invention provides a preparation method of nano-core-shell gallium indium zinc oxynitride. By using solvothermal-refrigeration-calcination technology, the metal precursor can be prepared into nano At the same time, creatively using the characteristic that In has a relatively faster evaporation rate than Zn, it is embedded in a large number of Zn vacancies in the surface layer during the nitriding process, thus forming a core-shell structure with different internal and external components. Calculations prove that the structure can promote the separation of photogenerated carriers, and then obtain excellent photocatalytic activity.
[0034] A method for preparing a gallium indium zinc ternary oxynitride with a core-shell structure, comprising the following steps:
[0035] (1) with Ga(NO 3 ) 3 ·xH 2 O, In(NO 3 ) 3 ·xH 2 O and Zn(Ac) 2 2H 2 O was used as the source of gallium, indium ...
Embodiment 1
[0040] This embodiment includes the following steps:
[0041] (1) 1.25mmol Ga(NO3)3 xH2O, 1.25mmol In(NO3)3 xH2O and 2.5mmol Zn(Ac)2 2H2O were added to reaction glass bottles filled with 4mL ethanolamine and 0.1mL acetic acid respectively, Using solvothermal method, keep at 60°C for 2 hours, transfer to corundum porcelain boat, seal with aluminum foil, transfer to low temperature (0-2°C) and keep for 1 week;
[0042] (2) Put the gel-like precursor obtained by keeping the low temperature for a long time in step (1) in a muffle furnace, raise the temperature to 500°C at a rate of 3°C / min, and bake at this temperature for 10h, and then follow the furnace Cool to room temperature to obtain light yellow GIZO fluffy powder;
[0043] (3) the GIZO powder that step (2) obtains is transferred in the tube furnace, in 200sccm ammonia gas (NH 3 ) at a flow rate of 5°C / min to 850°C and held for 10 hours, and then cooled to room temperature at a rate of 3°C / min to obtain GIZNO nanomaterial...
Embodiment 2
[0045] This embodiment includes the following steps:
[0046] (1) 1.25mmol Ga(NO 3 ) 3 ·xH 2 O, 1.25mmol In(NO 3 ) 3 ·xH 2 O and 2.5mmol Zn(Ac) 2 2H 2 O was added to reaction glass bottles filled with 4mL ethanolamine and 0.1mL acetic acid respectively, and kept at 50°C for 1h by solvothermal method, then transferred to a corundum porcelain boat, sealed with aluminum foil, and then transferred to a low temperature (0~ 2°C) for 1 week;
[0047] (2) Put the gel-like precursor obtained by keeping the low temperature for a long time in step (1) in a muffle furnace, raise the temperature to 600°C at a rate of 3°C / min, and bake at this temperature for 8 hours, and then follow the furnace Cool to room temperature to obtain light yellow GIZO fluffy powder;
[0048] (3) the GIZO powder that step (2) obtains is transferred in the tube furnace, in 200sccm ammonia gas (NH 3 ) at a flow rate of 5°C / min to a certain temperature T°C (T=600) for 12 hours, and then cooled to room tem...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Shell thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com