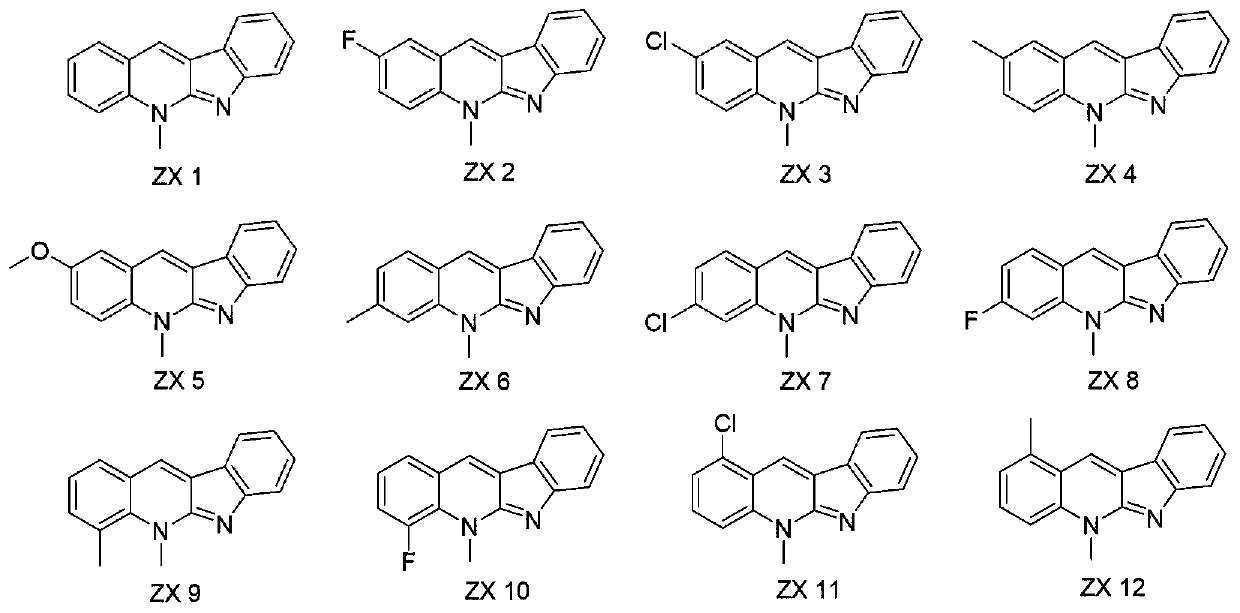
Application of A ring-modified new cryptolepine derivatives in prevention and control of agricultural plant diseases
A technology of selemenine and derivatives, applied in the field of natural medicinal chemistry, can solve problems such as product development stage, limited nutritional value and shelf life loss, etc., and achieve the effects of unique mode of action, high bactericidal activity, and human and animal safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
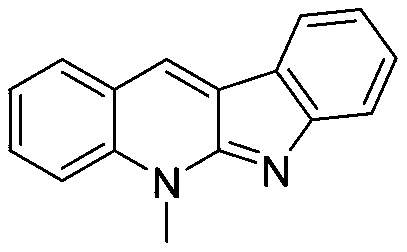
[0015] Embodiment 1: the synthesis of compound ZX-1
[0016]
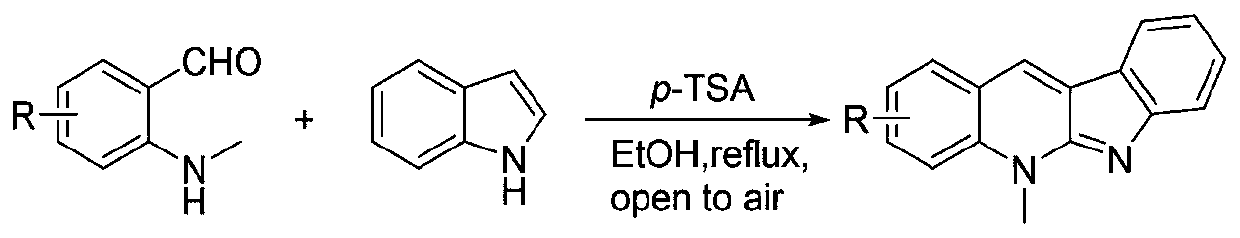
[0017] The synthetic method of compound ZX-1 of the present invention is carried out according to the following reaction formula:
[0018]
[0019] Synthesis of target compound ZX-1: Dissolve o-toluenebenzaldehyde (0.5mmol) in an appropriate amount of ethanol, then add indole (0.5mmol) and p-toluenesulfonic acid (0.5mmol), and heat to reflux for 24h. After cooling to room temperature, adjust the pH to alkaline with NaOH (1M) solution, then extract 3 times with dichloromethane, collect the organic phase and dry it with anhydrous magnesium sulfate, remove dichloromethane by rotary evaporation, column chromatography (dichloromethane methane / methanol=50:1) to obtain an orange-red solid, which is the target compound ZX1 (see literature for the synthesis method: Chemical Science, 2011, 2, 2178-2181).
[0020] Orange-red solid; Yield: 62%; m.p.105.32-106.54°C; 1 H NMR (400MHz, DMSO-d 6 )δ8.64(s,1H),8.03(d,J=7.5Hz...
Embodiment 2
[0021] Embodiment 2: the synthesis of compound ZX-2
[0022] The synthesis method is the same as in Example 1, except that 5-fluoro-2-(methylamino)benzaldehyde is used instead of o-toluidine.
[0023]
[0024] Orange-red solid; Yield: 70%; m.p.143.46-144.16°C; 1 H NMR (400MHz, DMSO-d 6 )δ8.85(s,1H),8.10(d,J=7.6Hz,1H),7.97(ddd,J=12.6,9.2,3.8Hz,2H),7.71(td,J=8.8,3.0Hz,1H ), 7.58(d, J=7.9Hz, 1H), 7.50(t, J=7.6Hz, 1H), 7.18(t, J=7.4Hz, 1H), 4.28(s, 3H). 13 C NMR (101MHz, DMSO-d 6 )δ156.04, 155.68, 133.82, 129.61, 128.51, 128.27, 123.89, 121.99, 121.28, 119.72, 119.20, 118.95, 117.63, 117.42, 114.44, 33.45. MS-ESI m / z: calcd for C 16 h 11 FN 2 :250.09[M+H] + .
Embodiment 3
[0025] Embodiment 3: the synthesis of compound ZX-3
[0026] The synthesis method is the same as in Example 1, except that 5-chloro-2-(methylamino)benzaldehyde is used instead of o-toluidine.
[0027]
[0028] Red solid; Yield: 50%; m.p.166.28-166.78°C; 1 H NMR (400MHz, DMSO-d 6 )δ8.83(d, J=5.5Hz, 1H), 8.18(t, J=3.5Hz, 1H), 8.10(d, J=7.5Hz, 1H), 7.96(d, J=9.0Hz, 1H) ,7.81(dt,J=6.7,3.3Hz,1H),7.59(d,J=7.9Hz,1H),7.50(t,J=7.6Hz,1H),7.19(t,J=7.4Hz,1H) ,4.26(d,J=4.1Hz,3H). 13 C NMR (101MHz, DMSO-d 6 )δ155.93, 155.62, 135.66, 130.57, 129.66, 128.80, 128.45, 128.06, 126.21, 124.09, 122.00, 121.71, 119.94, 117.78, 117.41, 33.37. MS-ESI m / z: calcd for C16 h 11 ClN 2 :266.06[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com