Prophylactic or therapeutic agent for organ fibrosis
A fibrosis and organ technology, applied in the field of cell preparation of pluripotent stem cells, to achieve the effect of improving function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0070] (2) Preparation and use of cell preparations
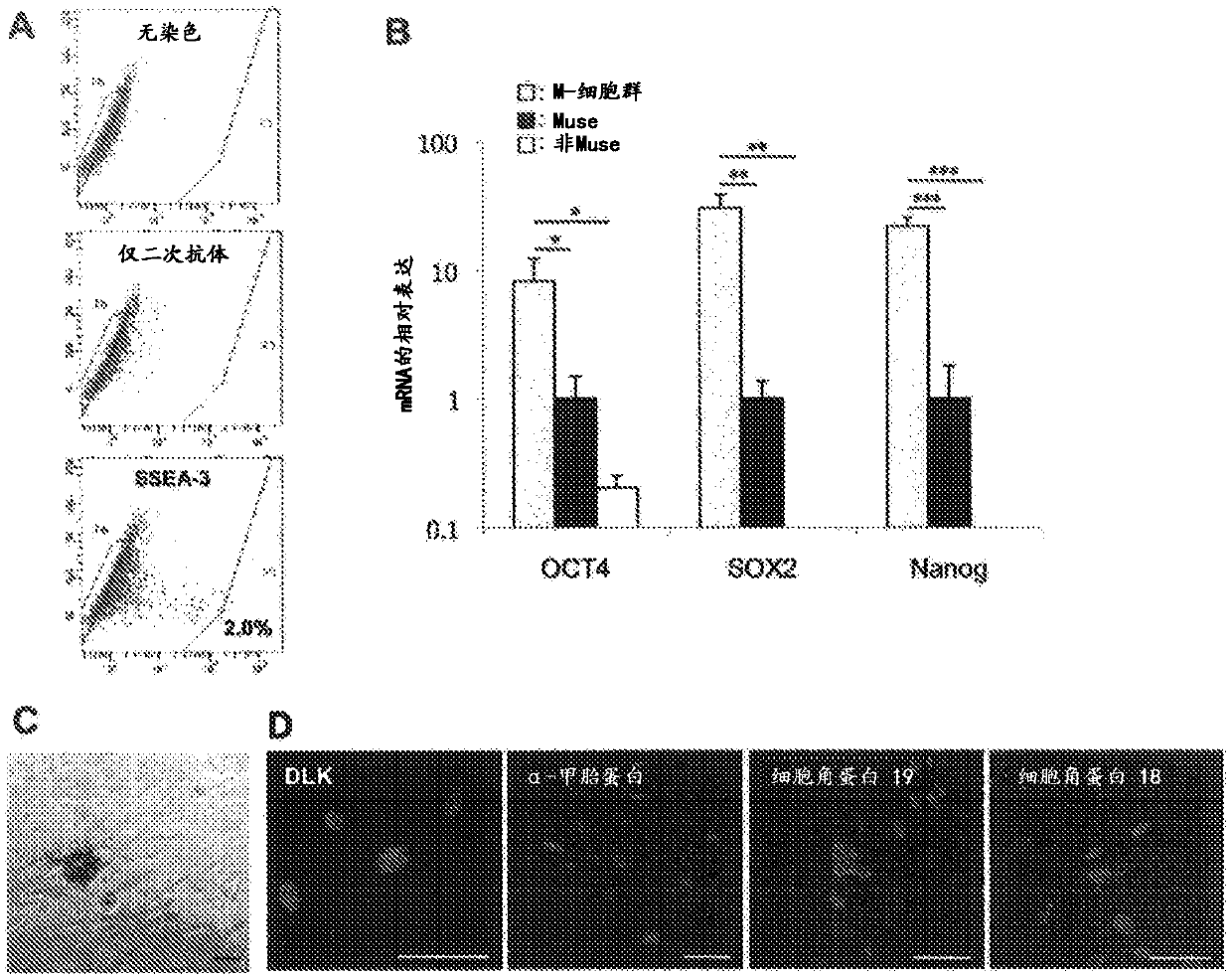
[0071] The cell preparation of the present invention is not limited, and can be obtained by suspending the Muse cells obtained in (1) above or a cell population containing Muse cells in physiological saline or an appropriate buffer (eg, phosphate-buffered saline). In this case, if the number of Muse cells isolated from autologous or non-autologous tissue is small, the cells may be cultured and proliferated before cell transplantation until a given number of cells can be obtained. It should be noted that, as already reported (International Publication No. WO2011 / 007900), Muse cells do not become tumors, so even if the cells recovered from the living tissue are directly contained in an undifferentiated form, the possibility of canceration Also lower is safe. In addition, the culture of recovered Muse cells is not particularly limited, and can be performed in a common growth medium (for example, α-minimal essential medium (α-...
Embodiment 1
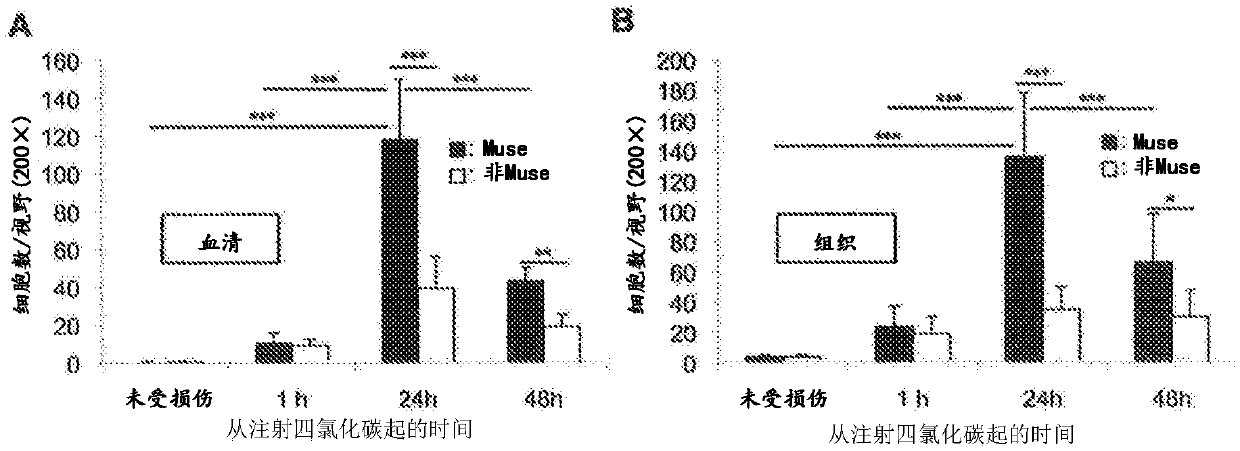
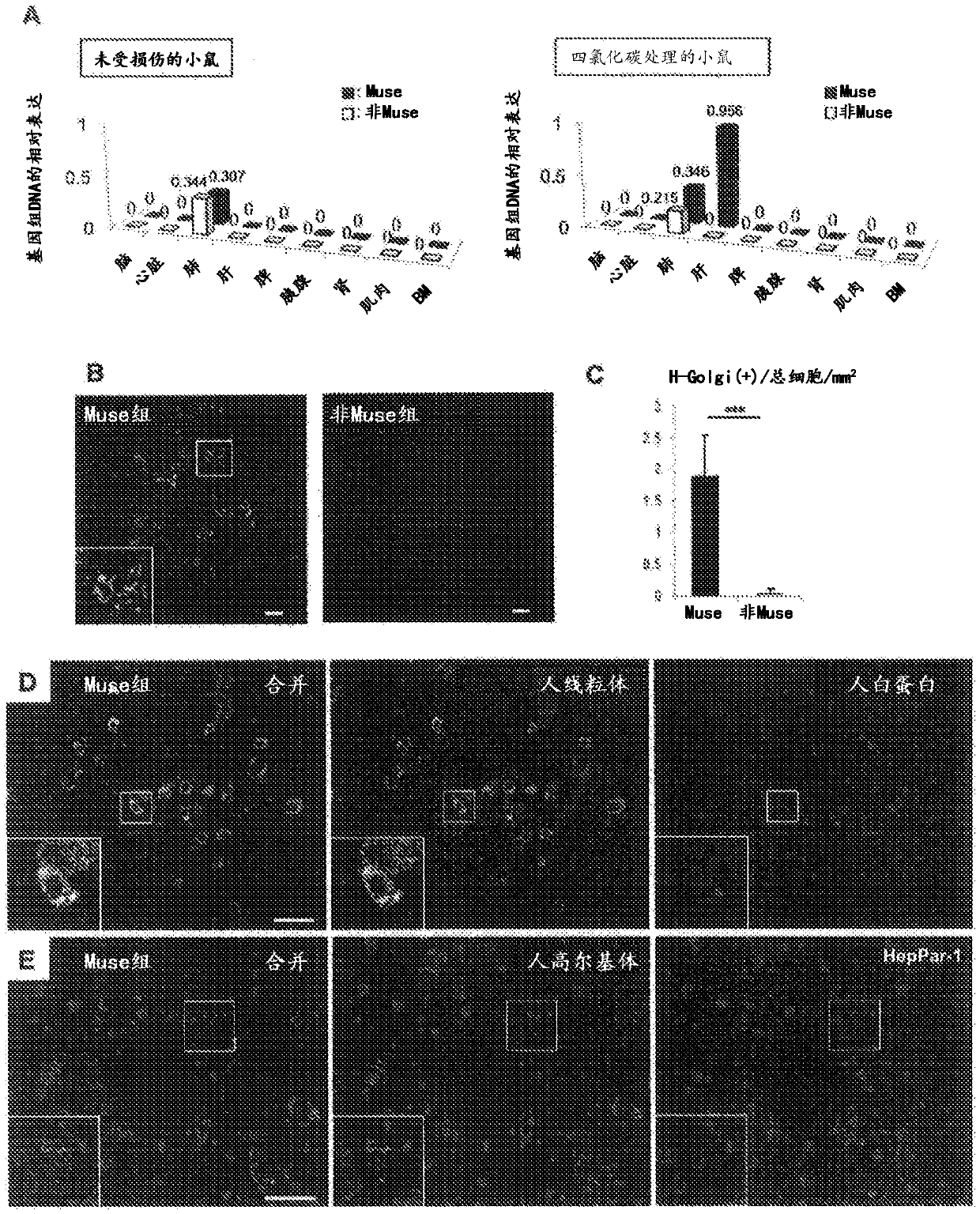
[0083] Transplantation of Human Muse Cells to Liver Fibrosis Model Mice
[0084] All studies use CB17 / Icr-Prkdc / CrlCrlj (SCID) mice. All animal experiments were performed in accordance with the guidelines for the humane care and use of experimental animals of Tohoku University (Sendai, Japan). The experimental procedures for making liver fibrosis models also refer to International Journal of Molecular Science 2012; 13:3598-3617 and Journal of Biochemistry 2003; 134:551-558. Specifically, by using CCl 4 (0.5ml / kg) intraperitoneal injection to make the liver fibrosis model of SCID mice.
[0085] Human Muse cells or non-Muse cells (5×10 4 cells) into the tail vein of liver fibrosis model mice.
[0086] Statistical Analysis
[0087] Significant differences between the two groups were assessed using the Student's t-test. Statistically significant differences between more than 3 groups were evaluated using one-way ANOVA with Bonferroni's multiple comparison test. Th...
Embodiment 2
[0112] Evaluation of Muse cells in skin fibrosis model
[0113] According to the method described in Sci Rep.2015Aug 20;5:12466.doi:10.1038 / srep12466., a BLM-induced skin fibrosis model mouse was produced. As mice, C57BL / 6J, female (Nippon SLC Co., Ltd.) was used. Physiological saline was administered instead of BLM in the control group.
[0114] On day 14 from BLM administration, 1 × 10 6 Cells / body weight (kg) of Muse cells. On the other hand, the vehicle administration group was administered the vehicle from the tail vein on day 14.
[0115] On day 28 from BLM administration, skin tissue was isolated, and collagen quantification, hematoxylin-eosin (HE) staining, and thickness analysis of the dermal layer were performed.
[0116] Skin collagen analysis was performed as described below.
[0117] Skin pieces were homogenized using 0.5M acetic acid / pepsin solution and stirred overnight at 4°C. Using the obtained extract, the skin collagen concentration was me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com