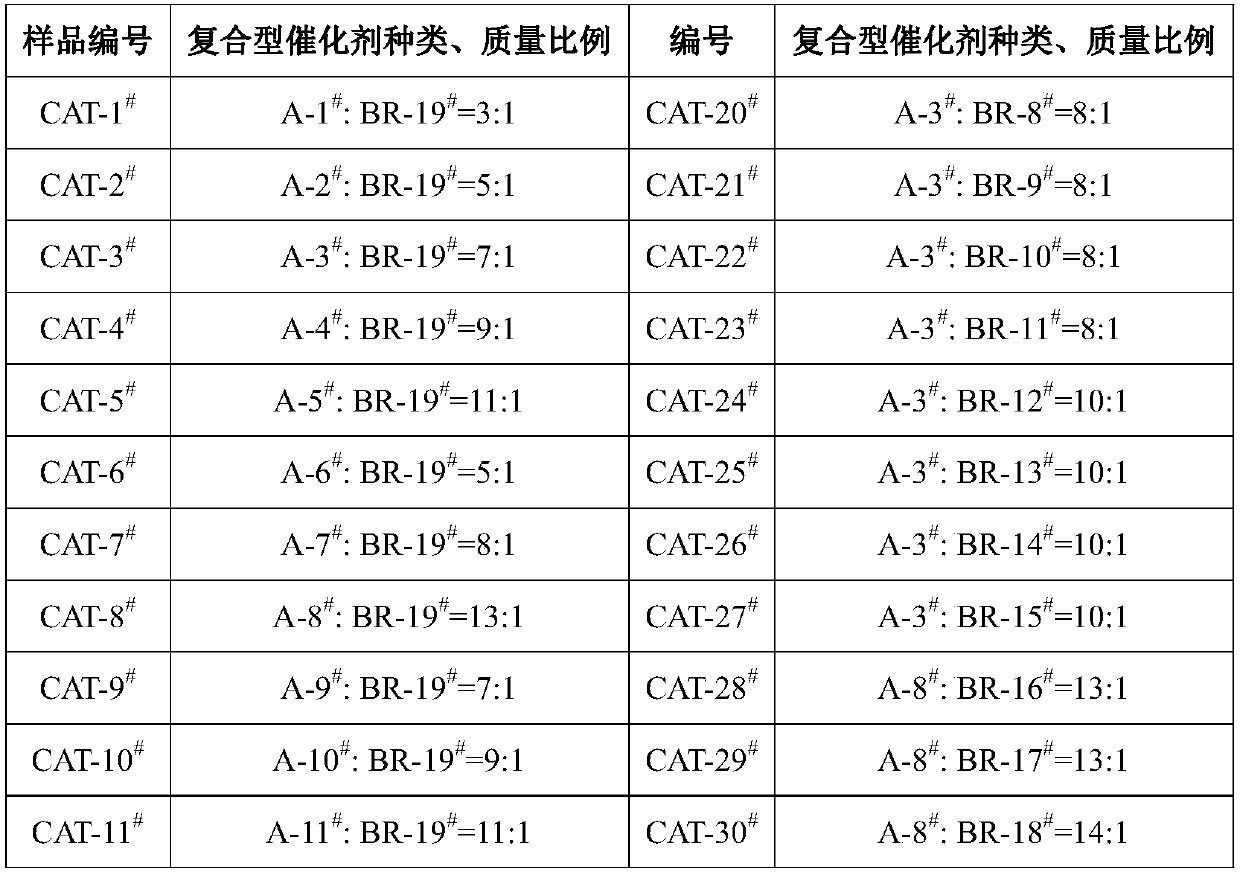
Composite type catalyst, preparation method of composite type catalyst and application in methylbenzene
A supported catalyst and a catalyst technology, applied in the field of catalysis, can solve the problems of low utilization rate of methanol, high ethylbenzene content, hindering the industrial application of the toluene-methanol side chain alkylation process, etc., and achieve the effect of improving the conversion rate of toluene
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
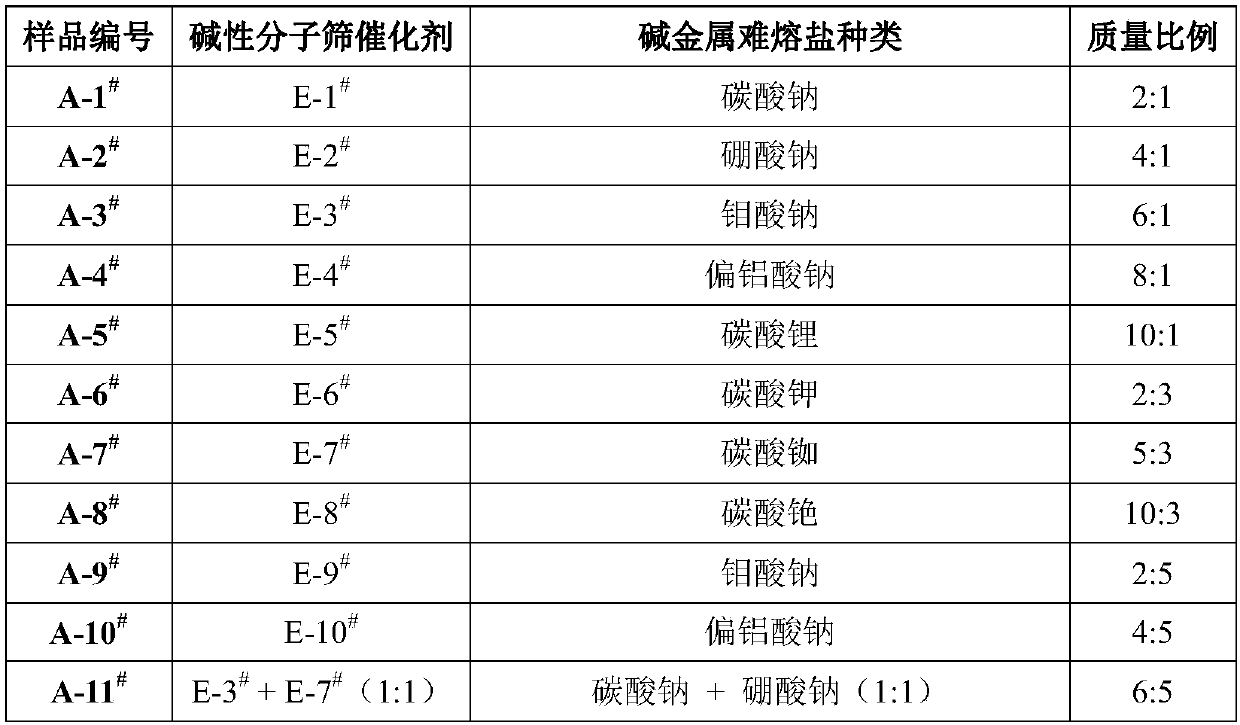
[0081] Embodiment 1 basic molecular sieve catalyst sample E-1 # ~E-10 # preparation of
[0082] Alkali metal ion exchanged molecular sieve sample E-1 # ~E-10 # The preparation comprises the following steps:
[0083] Weigh 30 g of molecular sieves, and perform ion exchange on the molecular sieves with the precursor solution. When the ion exchange solid-to-liquid ratio is 10:1, exchange at 80° C. for 4 hours. After suction filtration, washing and drying, the obtained solid was calcined in a muffle furnace at 550° C. for 6 hours. Repeat the above process twice, and the obtained sample is marked as E-1 # ~E-10 # .
[0084] The obtained sample number, precursor solution, solution concentration and ion exchange degree are shown in Table 1. Adopt XRF elemental analyzer to obtain sample E-1 # ~E-10 # Perform elemental analysis.
[0085] Table 1
[0086] Sample serial number
Embodiment 2
[0087] Embodiment 2 boron-containing catalyst sample BR-1 # ~BR-6 # preparation of
[0088] Boron-containing catalyst sample BR-1 # ~BR-6 # The preparation method is as follows:
[0089] Firstly, the boride is ground, then dried at 110°C and fired at 550°C for 6 hours. Obtain metal boride, sample number is BR-1 # ~BR-6 # .
[0090] The obtained sample numbers, types of metal borides and mixing ratios of metal borides are shown in Table 2. Wherein the mixing ratio is calculated according to the mass of the metal boride.
[0091] Table 2
[0092] Sample serial number
Embodiment 3
[0093] Embodiment 3 boron-containing catalyst sample BR-7 # ~BR-12 # preparation of
[0094] Boron-containing catalyst sample BR-7 # ~BR-12 # The preparation method is as follows:
[0095] Firstly, the borohydride compound is ground, then dried at 110°C and calcined at 550°C for 6 hours to obtain the borohydride compound, the sample number is BR-7 #~BR-12 # .
[0096] The obtained sample numbers, types of borohydride compounds, and mixing ratios of borohydride compounds are shown in Table 3. Wherein the mixing ratio is calculated according to the mass of the borohydride compound.
[0097] table 3
[0098] Sample serial number
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com