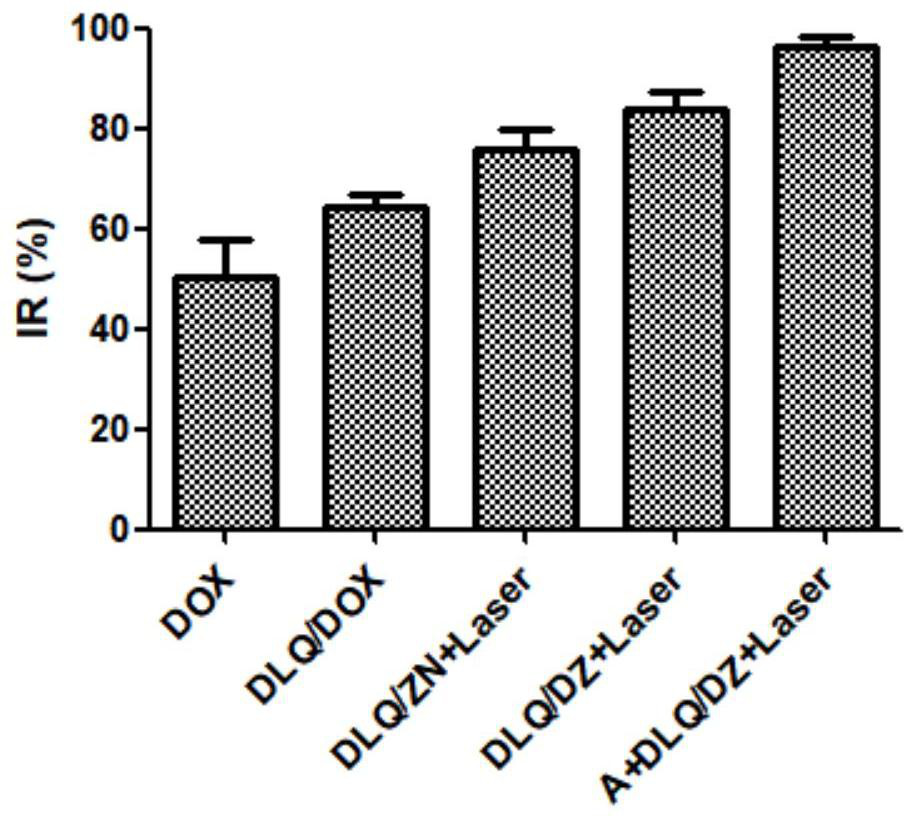
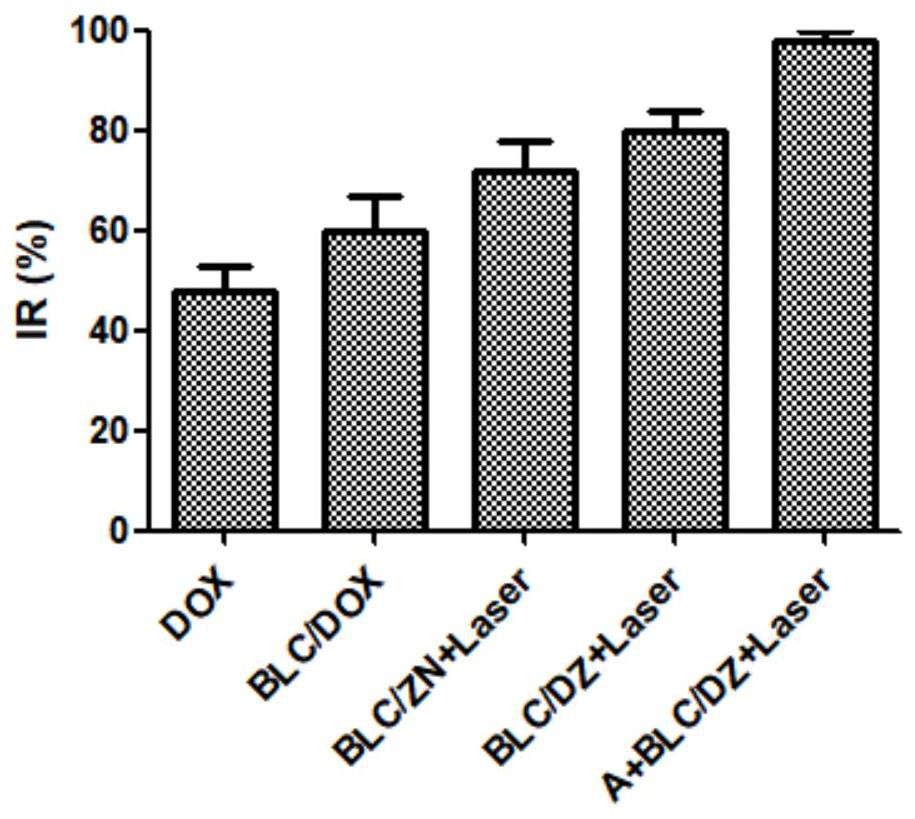
High-efficiency targeting conjugates triggered by biological clicks, multi-component compositions, preparation methods and applications thereof
A composition and conjugate technology, which is applied in the directions of drug combinations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. Limit the targeting ability and other issues to achieve the effect of reducing the frequency of administration, enhancing sensitivity and intake, and reducing toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Preparation of 5,6-dihydrodibenzo[b,f]azocyne-low molecular weight heparin-quercetin conjugate
[0044] Weigh 1 mmol of low molecular weight heparin and dissolve it in 10 mL of formamide, add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and N-hydroxysuccinimide under ice-bath condition, activate Low molecular weight heparin carboxyl. After activation in ice bath for 0.5 h, add 5 mL of quercetin solution dissolved in formamide (low molecular weight heparin: 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride: N-hydroxy Succinimide: the molar ratio of quercetin is successively 1: 4: 4: 2), slowly added dropwise in the low molecular weight heparin solution, after reacting for 24h, adding 5 times the volume of ice methanol for precipitation, suction filtration to obtain the precipitate, Reconstitute with an appropriate amount of distilled water, centrifuge at 3000 rpm for 10 min, ultrafilter, and freeze-dry to obtain a low molecular weight...
Embodiment 2
[0045] Example 2: Preparation of bicyclo[6.1.0]nonyne-unfractionated heparin-chrysin conjugate
[0046]Weigh 1 mmol of unfractionated heparin and dissolve it in 10 mL of N,N-dimethylformamide, and add N,N'-carbonyldiimidazole under ice-cooling conditions to activate the carboxyl group of unfractionated heparin. After activation in ice bath for 1 h, add 5 mL of chrysin solution dissolved in N,N-dimethylformamide (the molar ratio of unfractionated heparin:N,N'-carbonyldiimidazole:chrysin is 1:8:3) , slowly added dropwise to the unfractionated heparin solution, reacted for 48 hours, added 5 times the volume of glacial ether to precipitate, centrifuged to obtain the precipitate, redissolved with an appropriate amount of distilled water, centrifuged at 3000rpm for 10min, dialyzed in distilled water, and dried in vacuum to obtain the unfractionated Heparin-Chrysin Conjugate. Weigh an appropriate amount of unfractionated heparin-chrysin conjugate powder and dissolve it in 10 mL of f...
Embodiment 3
[0047] Example 3: Preparation of bicyclo[6.1.0]nonyne-desulfated heparin-curcumin conjugate
[0048] Weigh 1 mmol of desulfated heparin and dissolve it in 10 mL of N,N-dimethylacetamide, add N,N'-dicyclohexylcarboimide and 4-dimethylaminopyridine under ice-cooling conditions to activate the carboxyl group of desulfated heparin. After activation in ice bath for 2 h, add 5 mL of curcumin solution dissolved in a mixed solvent of formamide and N,N-dimethylacetamide (v:v=1:1) (desulfated heparin: N,N'- Hexylcarbimide: 4-dimethylaminopyridine: the molar ratio of curcumin is 1: 10: 10: 3), slowly added dropwise to the desulfated heparin solution, and after 36 hours of dark reaction, add 5 times the volume of Precipitate with ice methanol, filter with suction to get the precipitate, redissolve with appropriate amount of distilled water, centrifuge at 3000rpm for 10min, ultrafilter, and spray dry to obtain the desulfated heparin-curcumin conjugate. Weigh an appropriate amount of desul...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com