Method for preparing 3,4-dimethylpyrazole and phosphate and metal organic complex thereof
A technology of dimethylpyrazole and phosphoric acid, applied in the direction of 2/12 group organic compounds without C-metal bonds, zinc organic compounds, organic chemistry, etc., can solve the problem of inability to continuous production, complicated operation steps and low production efficiency and other problems, to achieve significant industrial application value, improve reaction efficiency, and reduce raw material costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
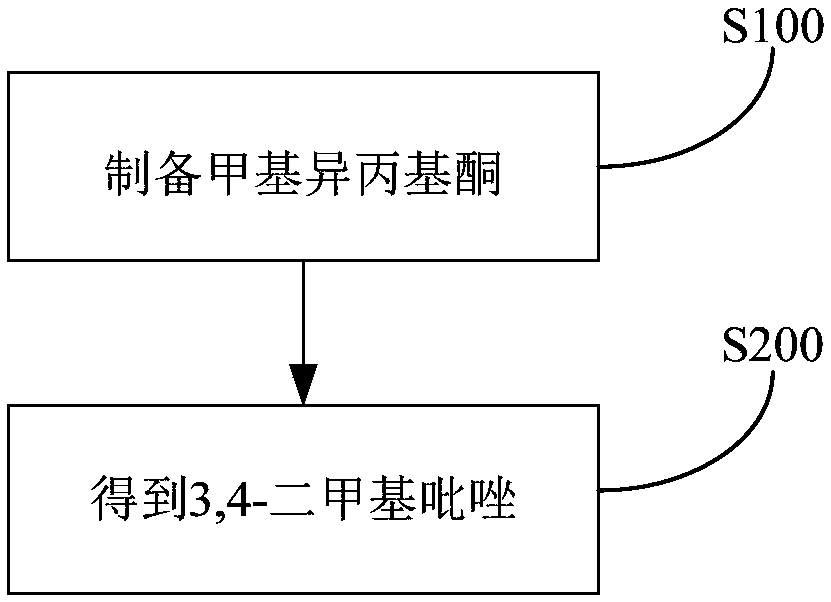
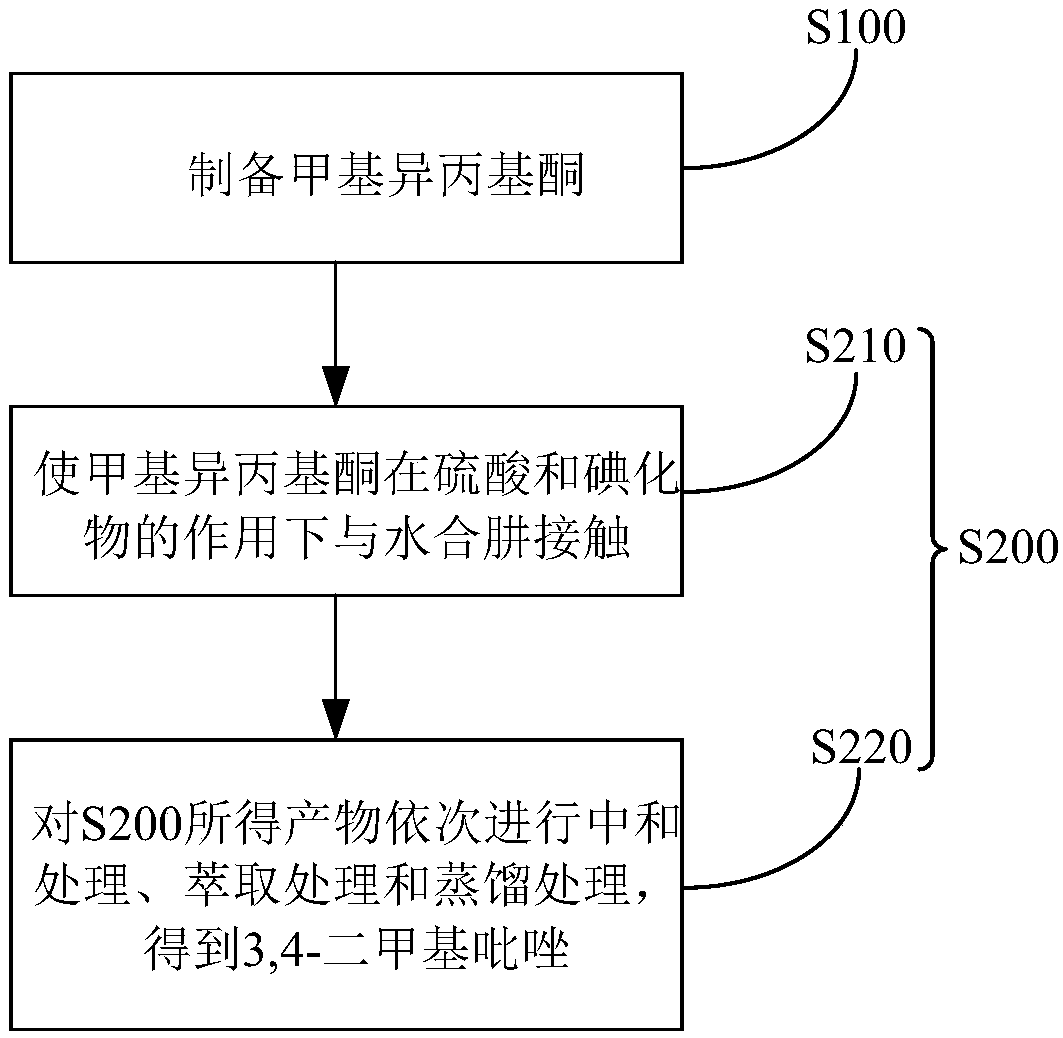
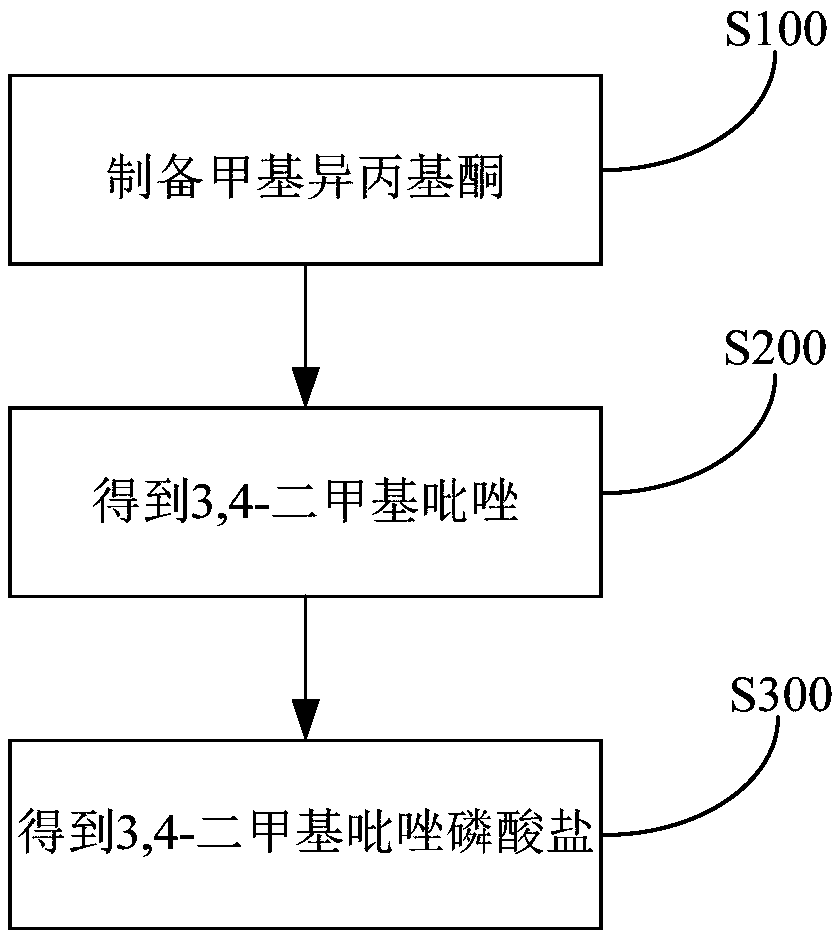
[0092] (1) Synthesis of methyl isopropyl ketone
[0093] After vaporizing and mixing water and isoprene separately, preheat to 180°C, enter H 3 PO 4 -B / diatomaceous earth catalyst bed for hydration reaction. The phosphoric acid content of the catalyst is 65wt%, the boron content is 6wt%, the reaction temperature is 250°C, the molar ratio of water and isoprene is 6.0, and the space velocity LHSV is 0.5h -1 , the reaction pressure is 0.1MPa. The reaction pressure was regulated by a pressure controller at the outlet of the reactor. The conversion rate of isoprene was 93.4%, the selectivity was 95.7%, the yield was 89.4%, and the purity of the product determined by gas chromatography was 99.7%.
[0094] (2) Synthesis of 3,4-dimethylpyrazole
[0095] Add 150kg (3kmole) of hydrazine hydrate with a mass fraction of 80% into the reactor, then add a small amount of water, start stirring, and slowly add 300kg (3kmole) of sulfuric acid with a concentration of 98% dropwise after the t...
Embodiment 2
[0098] Synthesis of 3,4-Dimethylpyrazole Phosphate (DMPP)
[0099] Dissolve 145kg of 3,4-dimethylpyrazole in 174kg of ethanol and react with 174kg of phosphoric acid with a mass fraction of 85%. The reaction temperature is 50°C and the reaction time is 8h. After the reaction is completed, the temperature is lowered, crystallized, and filtered , dried to obtain about 279.5kg of white powdery solid product, the yield was 95.4%, and the purity of the product as determined by high performance liquid chromatography was 99.3%.
Embodiment 3
[0101] Synthesis of 3,4-Dimethylpyrazole Complex with Zinc
[0102] After dissolving 96kg (1kmole) of 3,4-dimethylpyrazole in 200kg of chloroform, 100kg of absolute ethanol containing 34kg (250mole) of zinc chloride was added dropwise. Stir at room temperature for 8 hours, filter after the reaction is completed, wash the filter cake with methanol for 1 to 2 times, and obtain about 115kg of white powdery solid product after drying, with a yield of about 88%. The purity of the product determined by high performance liquid chromatography is 95.4%. The rate is 64% to 67.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com