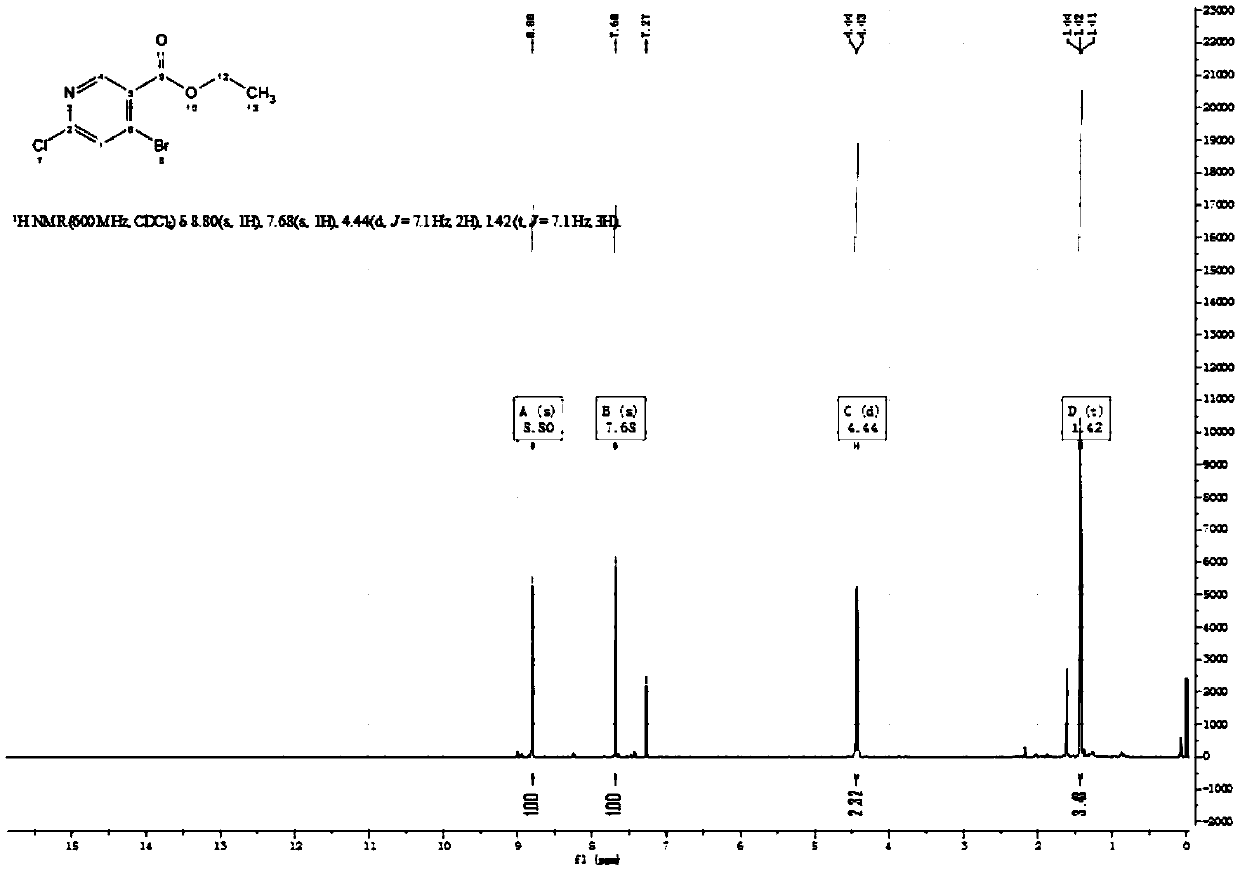
Synthetic method for 4-bromo-6-chloronicotinaldehyde
A synthetic method, the technology of chloronicotinic aldehyde, applied in the direction of organic chemistry, etc., can solve the problems of poor economic benefits and environmental impact, long process steps, complicated operation, etc., and achieve environmental friendliness, simplification of reaction process and post-treatment Process, the effect of optimizing the reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
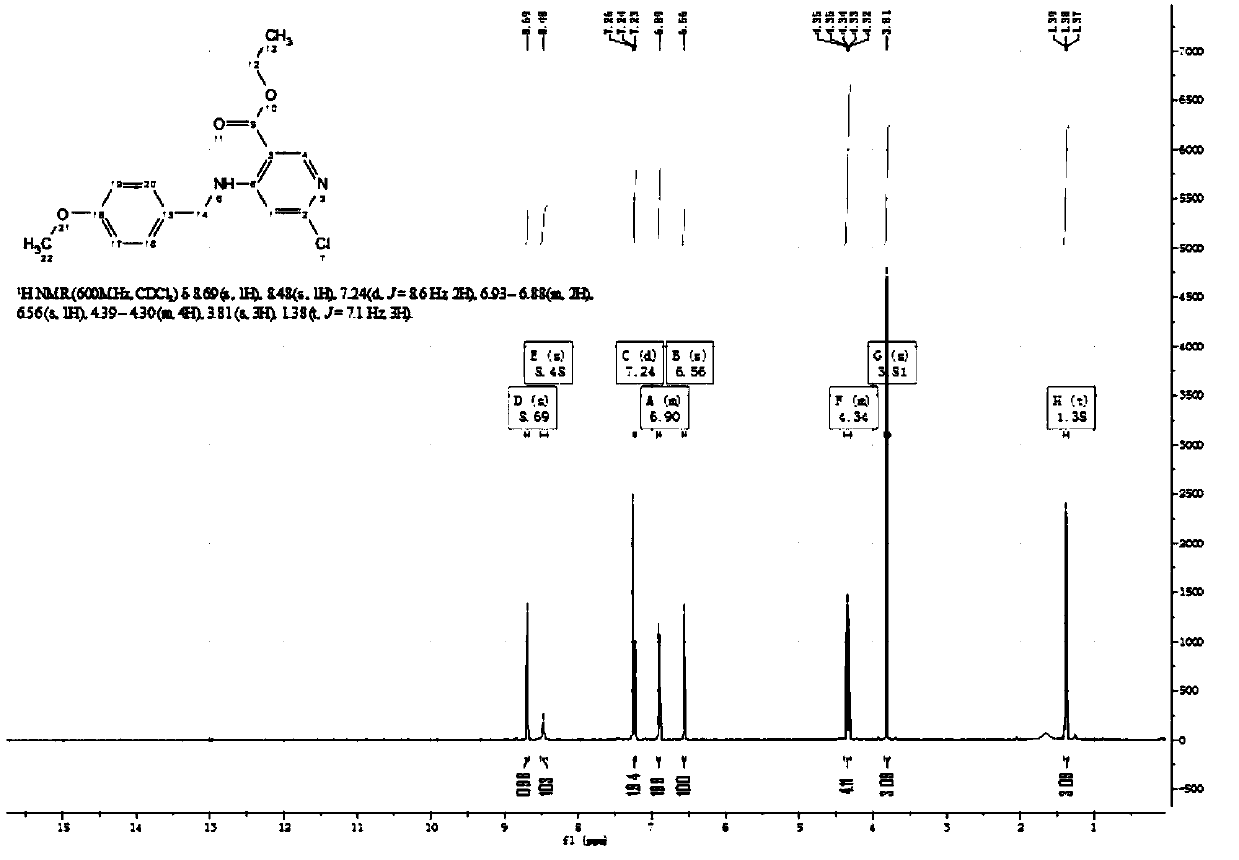
[0042] The first step is the synthesis of compound (1) ethyl 6-chloro-4-((4-methoxybenzyl)amino)nicotinate:
[0043] Add 150g of ethyl 4,6-dichloronicotinic acid (0.68mol) and 93.5g of 4-methoxybenzylamine (0.68mol) into a 3000ml dry three-necked flask, mechanically stir, heat to 40°C, stir and react overnight After the completion of the reaction detected by thin layer chromatography (TLC), it was added to ice water, extracted with ethyl acetate 3 times, backwashed with saturated brine, dried by adding anhydrous sodium sulfate, and the column was eluted [eluent : (Petroleum ether: ethyl acetate=40:1)] 196 g of compound (1) ethyl 6-chloro-4-((4-methoxybenzyl)amino)nicotinate was obtained, and the yield was 90%.
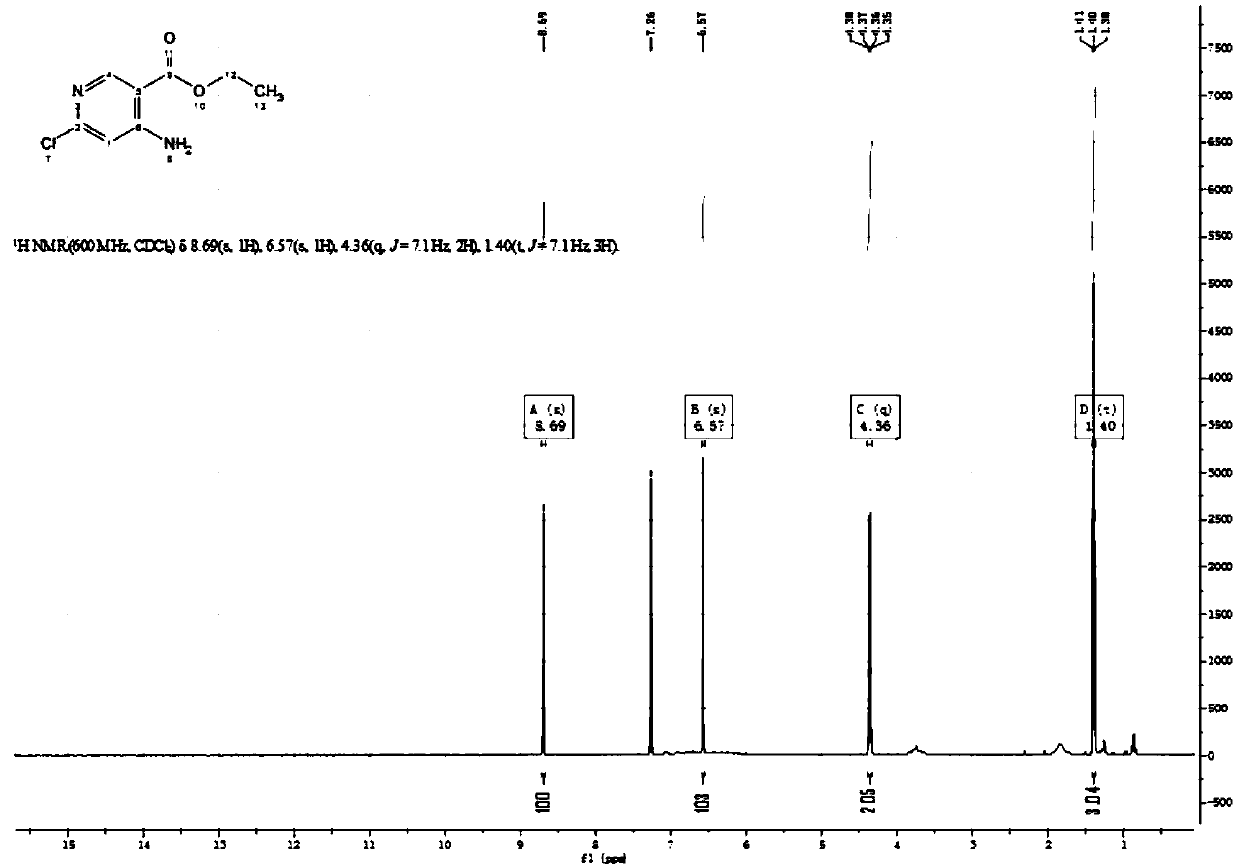
[0044] The second step is the synthesis of compound (2) ethyl 4-amino-6-chloronicotinate:
[0045] Add 196g of compound (1) ethyl 6-chloro-4-((4-methoxybenzyl)amino)nicotinic acid (0.61mol) to 1500ml of trifluoroacetic acid (20mol), and heat at 50℃~60℃ After reacting overni...
Embodiment 2
[0053] The first step is the synthesis of compound (1) ethyl 6-chloro-4-((4-methoxybenzyl)amino)nicotinate:
[0054] 150g of ethyl 4,6-dichloronicotinate (0.68mol) and 93.5g of 4-methoxybenzylamine (0.68mol) were added to a 3000ml dry three-necked flask, stirred mechanically, and reacted overnight at room temperature. According to TLC detection, the raw material did not react completely. It was added to ice water, extracted 3 times with ethyl acetate, backwashed with saturated brine, dried by adding anhydrous sodium sulfate, and the column layer was eluted [eluent: (petroleum ether: Ethyl acetate=40:1)] 120 g of compound (1) ethyl 6-chloro-4-((4-methoxybenzyl)amino)nicotinic acid was obtained, and the yield was 55%.
[0055] The second step is the synthesis of compound (2) ethyl 4-amino-6-chloronicotinate:
[0056] 120g compound (1) ethyl 6-chloro-4-((4-methoxybenzyl)amino)nicotinic acid (0.37mol) was added to 600ml trifluoroacetic acid (8.07mol) and heated at 50℃~60 React overnigh...
Embodiment 3
[0064] The first step is the synthesis of compound (1) ethyl 6-chloro-4-((4-methoxybenzyl)amino)nicotinate:
[0065] Add 150g of ethyl 4,6-dichloronicotinate (0.68mol) and 187g of 4-methoxybenzylamine (1.36mol) into a 3000ml dry three-necked flask, mechanically stir, heat to a reaction temperature of 60℃, and stir to react Overnight, TLC detection showed that the reaction was complete, added to ice water, extracted with ethyl acetate 3 times, backwashed with saturated brine, dried with anhydrous sodium sulfate, and the column layer was eluted [eluent: (petroleum ether: Ethyl acetate=40:1)] to obtain 109 g of compound (1) ethyl 6-chloro-4-((4-methoxybenzyl)amino)nicotinate with a yield of 50%.
[0066] The second step is the synthesis of compound (2) ethyl 4-amino-6-chloronicotinate:
[0067] Add 109g of compound (1) 6-chloro-4-((4-methoxybenzyl)amino)nicotinic acid ethyl ester (0.34mol) to 1010ml of trifluoroacetic acid (13.59mol), and heat at 50℃~60 React overnight at ℃, check by ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com