High-efficiency catalyst for preparing 1,3-butadiene by using carbon dioxide to oxidize 1-butene to dehydrogenate and preparation method thereof
A carbon dioxide and catalyst technology, applied in the field of high-efficiency loaded iron-based composite oxide catalyst and its preparation, can solve the problems of poor stability and low catalyst activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~7
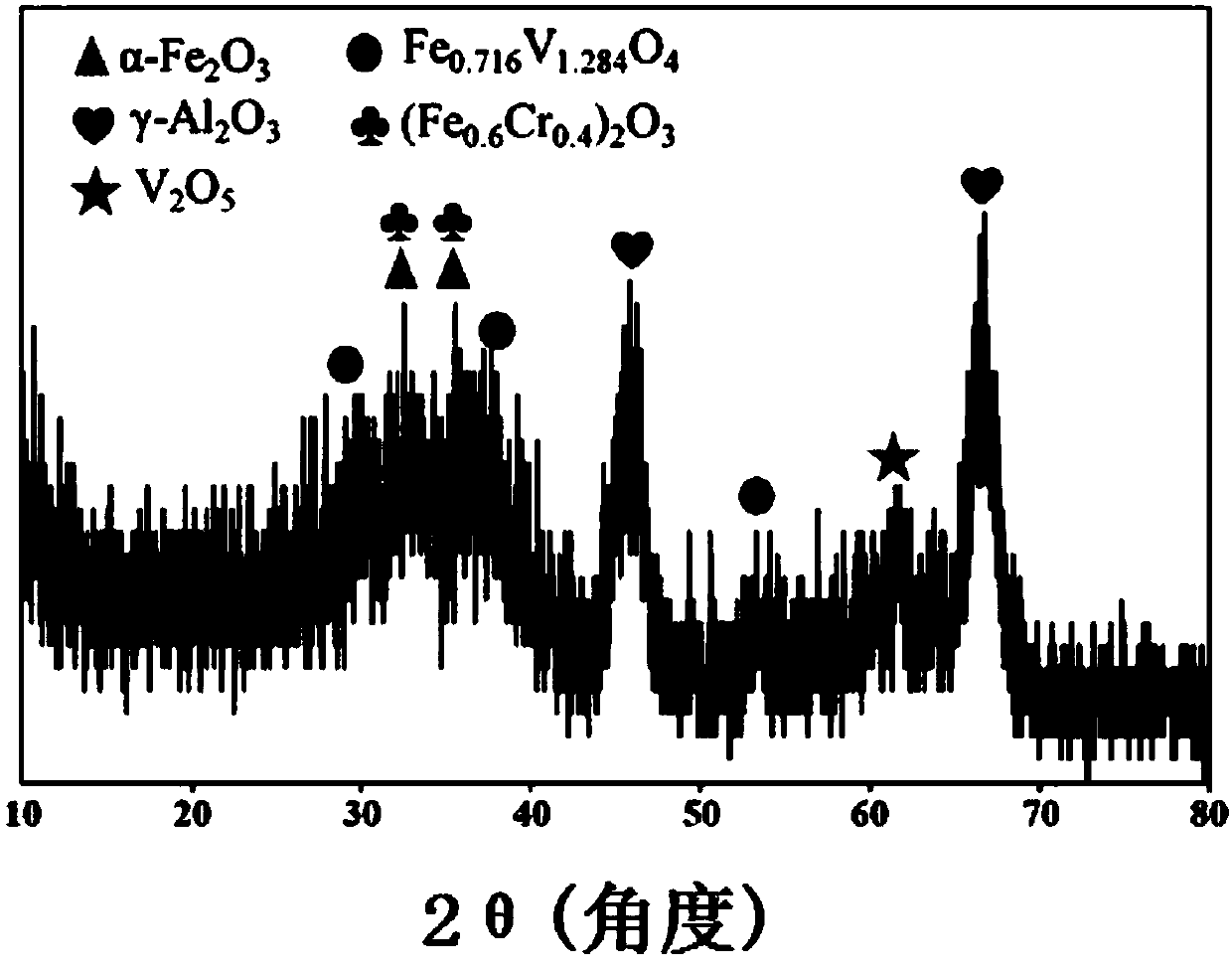
[0032] In traditional Fe 2 o 3 / Al 2 o 3 On the basis of the catalyst, a single element doping modification is carried out, wherein the doping element is selected from one of Co, Cr, Cu, Mn, Ni, V or Zn. The specific preparation method is as follows:
[0033] Weigh γ-Al 2 o 3 3g, Fe(NO 3 ) 3 9H 2 O 3.2464g (0.0080mol), nitrates of doping elements (wherein V is NH 4 VO 3 ) 0.00089mol, that is, the molar ratio of Fe to doping elements is 9:1, measure 100mL of distilled water, put it in an eggplant-shaped bottle at 60°C and continuously stir for 4h. After the water solvent was removed by rotary evaporation, it was dried in an oven at 120°C for 4 hours. Then put the above samples in a muffle furnace and bake them at 600°C for 4h (heating rate 5°C / min) to obtain the samples, respectively denoted as FeCoO x / Al 2 o 3 , FeCrO x / Al 2 o 3 , FeCuO x / Al 2 o 3 , FeMnO x / Al 2 o 3 , FeNiO x / Al 2 o 3 、FeVO x / Al 2 o 3 , FeZnO x / Al 2 o 3 .
Embodiment 8
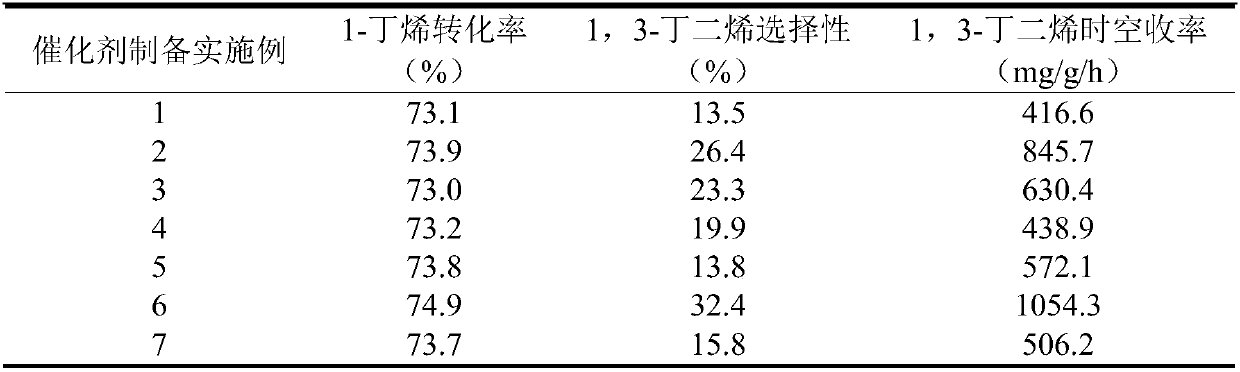
[0035] Catalyst activity evaluation was carried out in the atmospheric pressure micro-reaction system, and the reactants were fed 6mL / min 1-butene and 54mL / min CO 2 , that is, the ratio of intake air to CO 2 / C 4 h 8 =9:1, using 0.2g of the catalysts of Examples 1 to 7 respectively, that is, the space velocity is 4.5h -1 , the reaction was carried out at 600°C under normal pressure, and the product was analyzed by gas chromatography. Carry out 1-butene conversion rate when carrying out 10min with reaction, the selectivity of 1-butene to 1,3-butadiene and the space-time yield of 1,3-butadiene are index, and gained reaction performance is as shown in table 1 . It can be clearly seen from the results that the selectivity of 1,3-butadiene can be effectively improved by doping V elements, thereby increasing the space-time yield of 1,3-butadiene, and the highest space-time yield of 1,3-butadiene 1054.3 mg / g / h. Therefore, V element is selected as the first element to be doped. ...
Embodiment 9~10
[0039] Under the reaction condition of embodiment 8, adopt the catalyzer among the embodiment 6 (FeVO x / Al 2 o 3 ) to investigate the reaction temperature in the reaction conditions of synthesizing 1,3-butadiene, to explore the optimum reaction temperature. Set the reaction temperature as 500°C and 550°C respectively. Carry out 1-butene conversion ratio when carrying out 10min with reaction, 1-butene is to 1, the selectivity of 3-butadiene and the space-time yield of 1,3-butadiene as index, and gained reaction performance is as shown in table 2 . It can be seen from Table 2 that the activity of the catalyst is not as high as that at 600° C. (the reaction conditions of Example 8) when the reaction temperature is 500° C. or 550° C. Therefore, 600°C is the optimal temperature for the reaction.
[0040] Table 2 FeVO at different reaction temperatures x / Al 2 o 3 Catalytic performance of the catalyst
[0041]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com