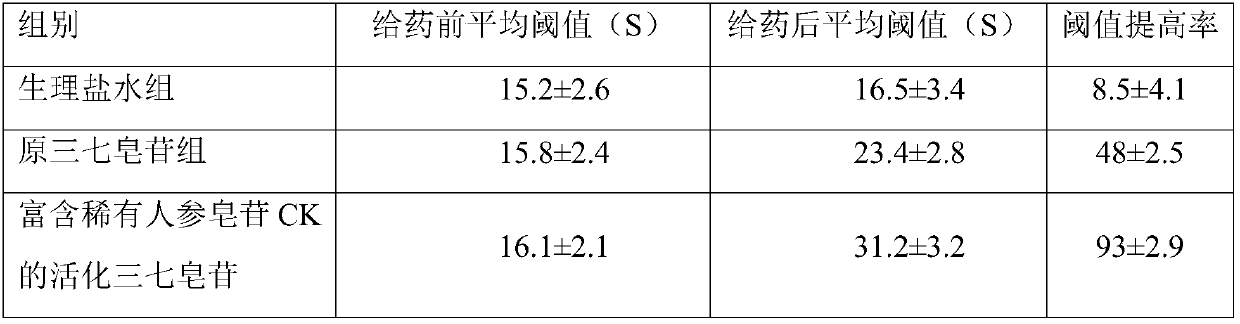
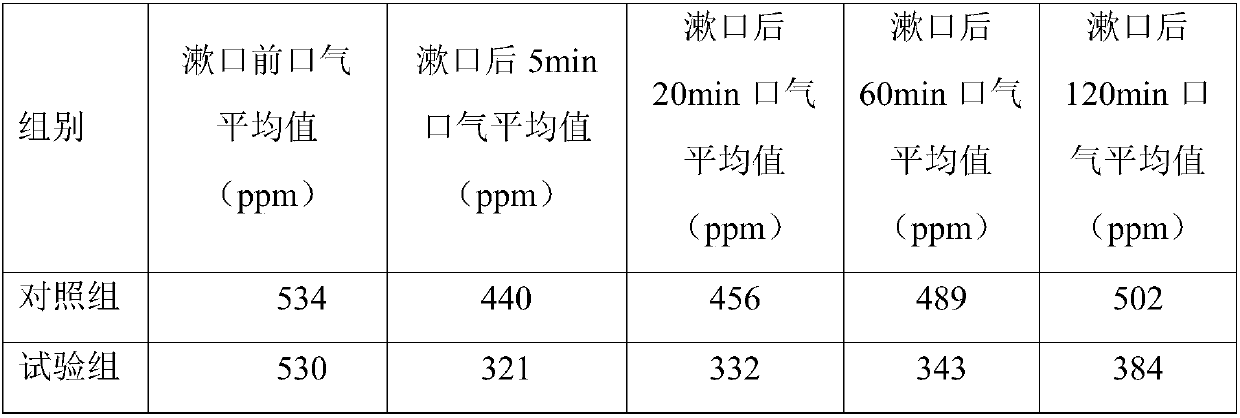
Application of activated notoginsenoside rich in rare ginsenoside in preparation of oral care products
A technology for ginsenosides and activation of Panax notoginseng, which is applied to medical preparations containing active ingredients, cosmetic preparations, oral care and other directions, can solve the problems of poor membrane permeability of Panax notoginseng saponins, difficulty in exerting medicinal effects, and poor human absorption. , to avoid discoloration, reduce bleeding gums, and promote periodontal health
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] A toothpaste rich in activated notoginsenosides of rare ginsenosides, the weight percentage of each component is:
[0055] Rare ginsenoside-rich activated notoginseng saponin 0.1%, calcium carbonate 50%, polyethylene glycol 2%, sorbitol 18%, carmellose sodium 0.8%, xanthan gum 0.4%, sodium lauryl sulfate 2.5%, Cocamidopropyl Betaine 0.5%, Sodium Pyrophosphate 0.2%, Sodium Saccharin 0.25%, Methylparaben 0.15%, Propylparaben 0.05%, Fragrance 1.2%, Deionized water added to 100 %.
[0056] Among them, the raw material components in this example: the preparation process of activated notoginseng saponins rich in rare ginsenosides is as follows:
[0057] 1) Extract the total saponins of Panax notoginseng by using the root of Panax notoginseng as raw material: take the dried Panax notoginseng root, crush it, pass through a 100-mesh sieve, extract with 95% ethanol under reflux, filter, and concentrate the filtrate under reduced pressure to obtain the extract of Panax notoginsen...
Embodiment 2
[0068] A toothpaste rich in activated notoginsenosides of rare ginsenosides, the weight percentage of each component is:
[0069] Rare ginsenoside-rich activated notoginseng saponin 0.3%, calcium hydrogen phosphate 35%, silicon dioxide 5%, propylene glycol 5%, glycerin 10%, sorbitol 15%, sodium carboxymethylcellulose 0.5, guar gum 0.5 %, sodium lauryl sulfate 2.3%, sodium lauroyl sarcosinate 0.5%, sodium pyrophosphate 0.2%, sucralose 0.22%, sodium benzoate 0.3%, essence 1.0%, deionized water added to 100%.
[0070] Among them, the raw material components in this embodiment: the preparation process of activated notoginseng saponins rich in rare ginsenosides is as follows:
[0071] 1) Using the stems and leaves of Panax notoginseng as raw materials to extract the total saponins of Panax notoginseng: take the dried stems and leaves of Panax notoginseng, crush them, pass through a 100-mesh sieve, extract with 70% ethanol under reflux, filter, and concentrate the filtrate under red...
Embodiment 3
[0082] A toothpaste rich in activated notoginsenosides of rare ginsenosides, the weight percentage of each component is:
[0083] Rare ginsenoside-rich activated notoginseng saponin 0.5%, silicon dioxide 18%, polyethylene glycol 4%, sorbitol 56%, sodium carboxymethylcellulose 0.6, carrageenan 0.2%, sodium lauryl sulfate 2 %, sodium pyrophosphate 0.5%, aspartame 0.05%, stevia 0.05%, sodium benzoate 0.3%, essence 0.8%, titanium dioxide 0.2%, deionized water added to 100%.
[0084] Among them, the raw material components in this example: the preparation process of activated notoginseng saponins rich in rare ginsenosides is as follows:
[0085] 1) Extract the total saponins of Panax notoginseng by using the root of Panax notoginseng as raw material: take the dried Panax notoginseng root, crush it, pass through a 100-mesh sieve, extract with 80% ethanol under reflux, filter, and concentrate the filtrate under reduced pressure to obtain the extract of Panax notoginseng; Use supercr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com