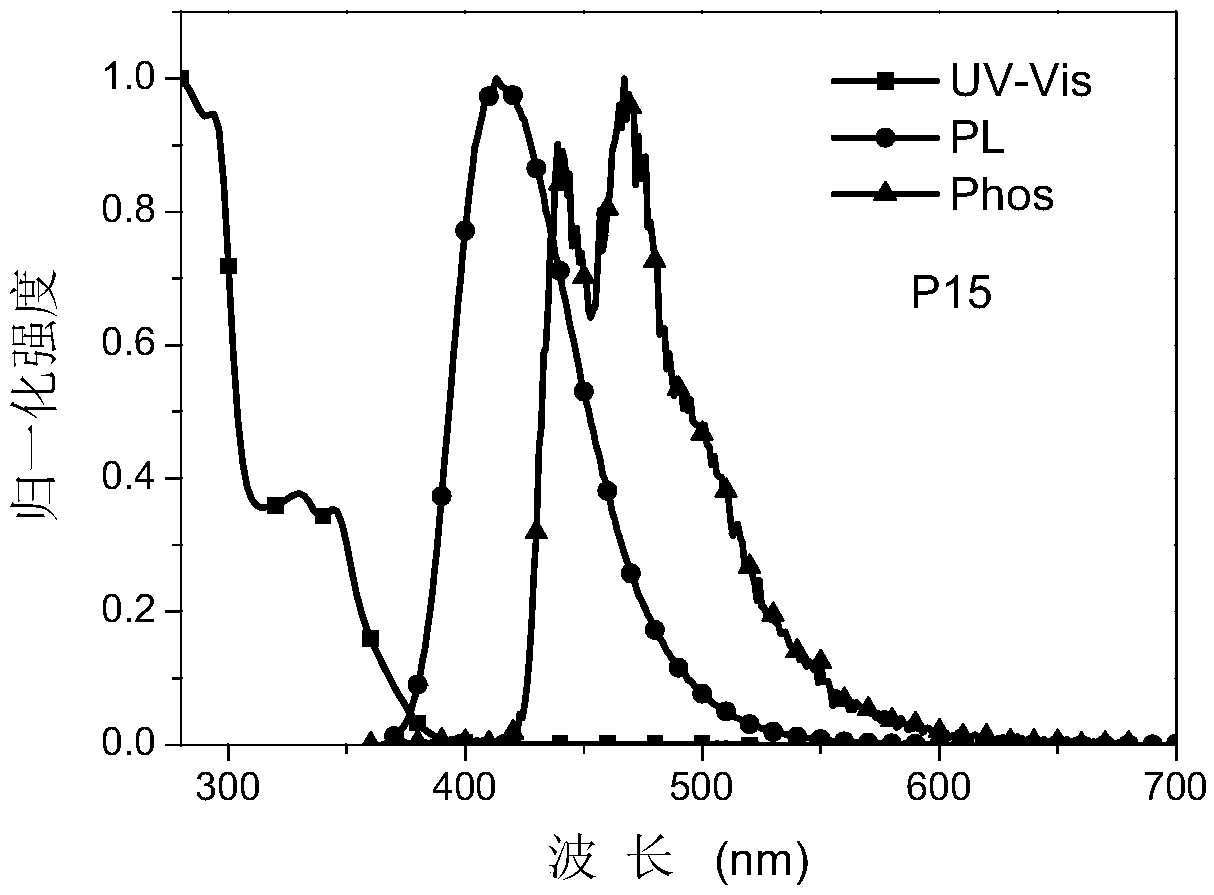
Organic photoelectric material based on nitrogen-heterocyclic molecules substituted by carbazole derivatives and preparation method and application of organic photoelectric material
A technology of organic optoelectronic materials and carbazole derivatives, which can be used in organic chemistry, circuits, electrical components, etc., and can solve the problems of ineffective use of triplet exciton energy, low exciton radiation deactivation rate, and weak coupling effect. , to achieve the effect of simple structure, strong electrochemical stability and stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
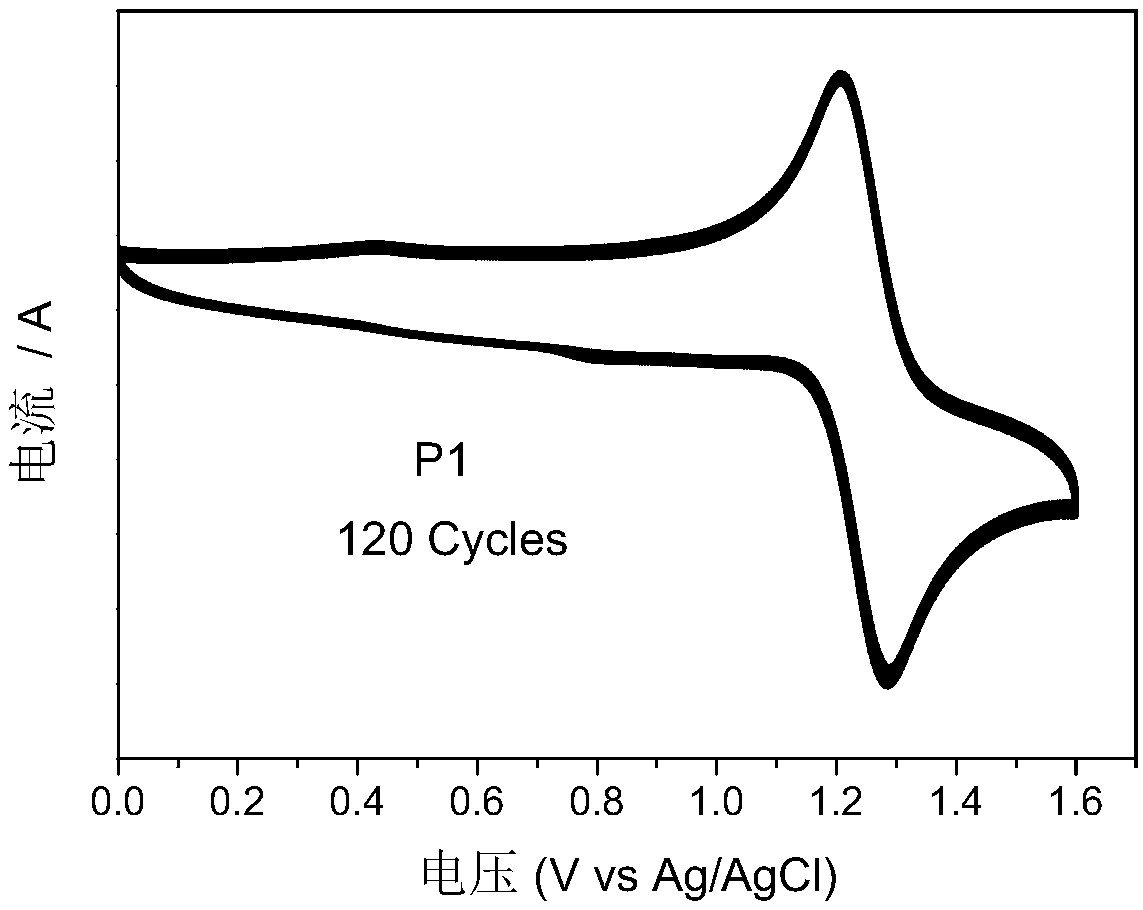
[0045] In this embodiment, intermediate a is first prepared, and the specific synthesis steps are shown in the following formula:
[0046]
[0047] in N 2 Atmosphere and a low temperature environment of -78°C, in a 100mL three-necked flask, 2.03g (10mmol) of 5-bromo-2-fluoro-1,3-dimethylbenzene was dissolved in 60mL of anhydrous tetrahydrofuran THF, and then 6.0 mL of 2.5 mol / L n-butyllithium (n-hexane as solvent) was added dropwise, and the system was kept at -78°C and stirred for 1 h. Afterwards, 4.1 mL (20 mmol) of isopropanol pinacol borate was slowly added to the reaction system, and the reaction was continued to be stirred at -78°C for 1 h, and then the system was returned to room temperature for overnight reaction. After the reaction was completed, THF was removed by rotary evaporation, extracted several times with dichloromethane DCM and water, the organic phase was taken out, excess DCM was removed by rotary evaporation, and 1.75 g of intermediate a was obtained b...
Embodiment 2
[0055] The organic photoelectric material P2 based on nitrogen heterocyclic molecules substituted by carbazole derivatives, the specific synthesis steps are shown in the following formula:
[0056]
[0057] The 3,6-di-tert-butylcarbazole used in the preparation of P1 in Example 1 was replaced with 999.94 mg (4.4 mmol) of equivalent 3,6-dimethoxycarbazole, and the rest of the raw materials and steps were the same as in Example 1. Likewise, 1.22 g of product P2 can be obtained with a yield of 75%. Molecular formula: C 54 h 46 N 4 o 4 ; M / Z=814.35; m / s=814.35 (100.0%), 815.36 (58.4%), 816.36 (16.7%), 817.36 (2.3%); Elemental analysis: C, 79.58; H, 5.69; N, 6.87; O, 7.85.
Embodiment 3
[0059] Firstly, intermediate B is prepared, and the specific synthesis steps are shown in the following formula:
[0060]
[0061] The 4,6-dichloro-2-phenylpyrimidine used in the preparation of A intermediate in Example 1 was replaced by an equivalent of 2,4-dichloro-6-phenyl-1,3,5-triazine 565.15mg (2.5mmol), all the other raw materials and steps are the same as in Example 1, and 883mg of intermediate B can be obtained with a yield of 88%. Molecular formula: C 25 h 21 f 2 N 3 ; M / Z=401.17; m / s=401.17 (100.0%), 402.17 (27.0%), 403.18 (2.7%), 402.17 (1.1%); Elemental analysis: C, 74.80; H, 5.27; F, 9.46; N, 10.47.
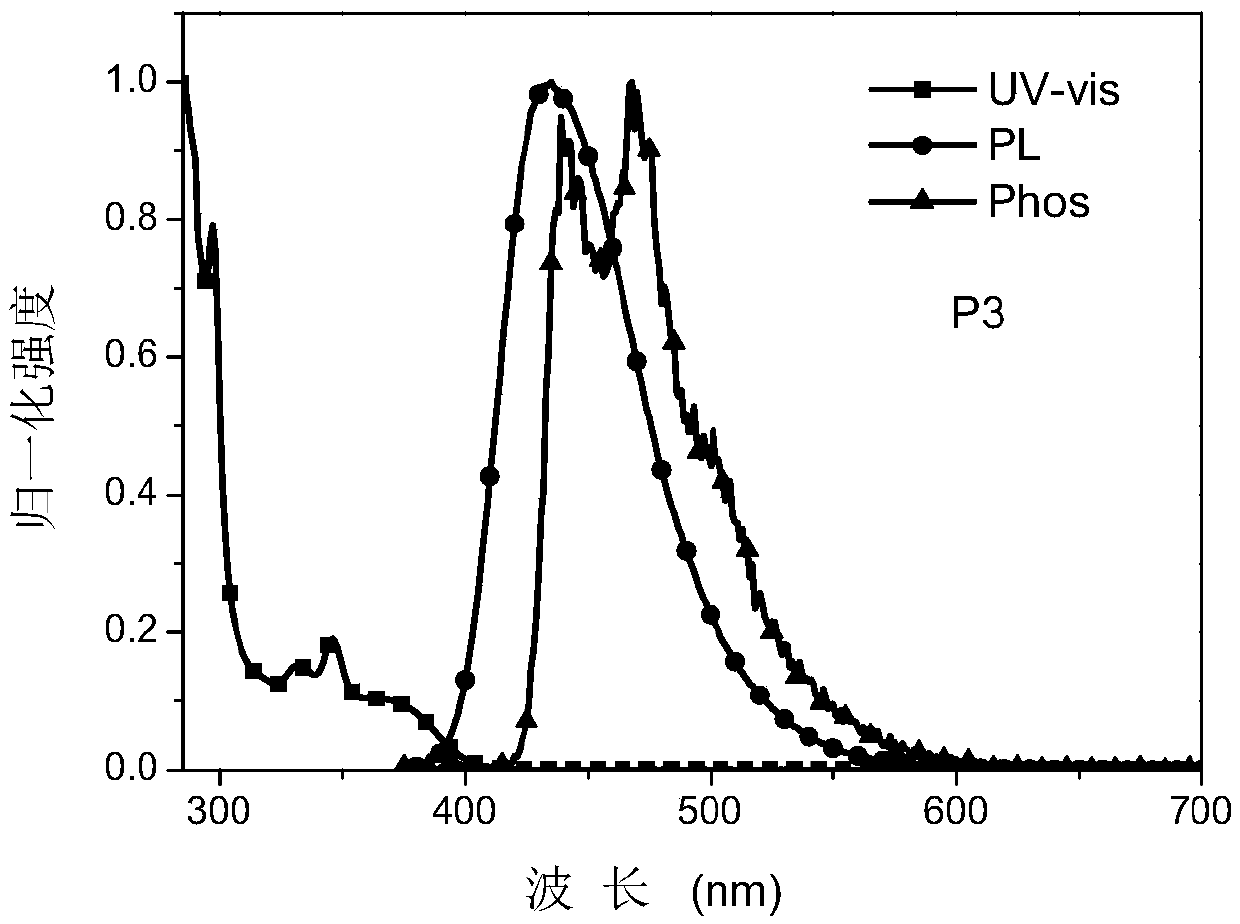
[0062] The organic photoelectric material P3 based on nitrogen heterocyclic molecules substituted by carbazole derivatives, the specific synthesis steps are shown in the following formula:
[0063]
[0064] The A intermediate used in the preparation of P1 in Example 1 was replaced by an equivalent of 802.92 mg (2 mmol) of B intermediate, and the rest of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com