Multifunctional triarylamine-containing oxazine polymer and preparation method and application thereof
A triarylamine-based, polymer technology, applied in chemical instruments and methods, through chemical reaction of materials for analysis, fluorescence/phosphorescence, etc., can solve the problems of poor film adhesion and poor heat resistance, etc. Effects of solubility, good heat resistance, excellent electrochromic properties and memory properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
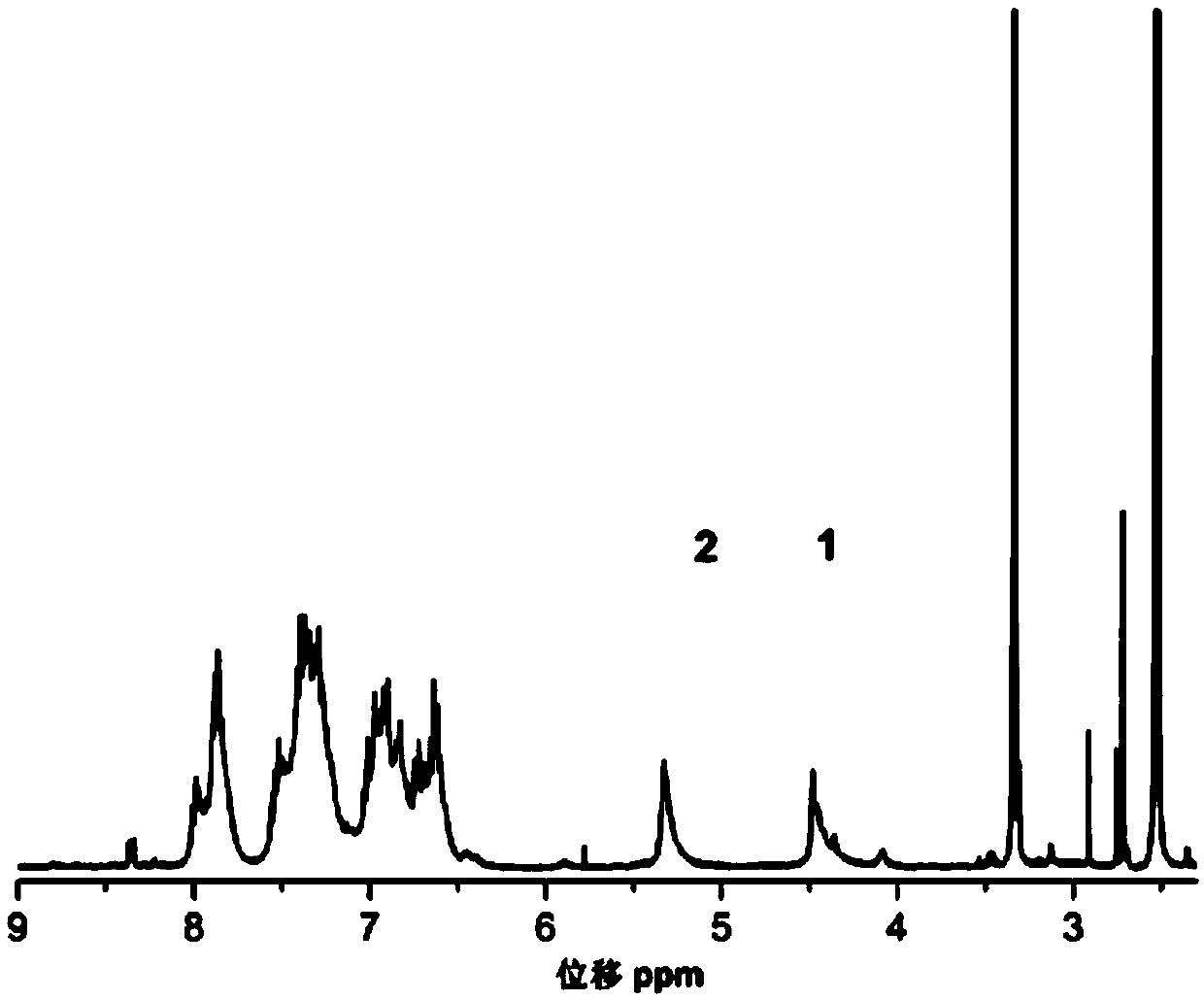
[0032] Specific embodiment 1: The structural formula of a kind of multifunctional triarylamino-containing oxazine polymer in this embodiment is as follows: In the formula, n is an integer of 3 to 10.
[0033] In this embodiment, the triphenylamine-based monomer is prepared first, and this embodiment uses N 4 ,N 4 -Di-naphthalene-2-yl-biphenyl-4,4-diamine is used as raw material and then reacted with p-fluoronitrobenzene to realize nitration, and then the nitro group is reduced to amino, with bisphenol A, paraformaldehyde , the prepared triphenylamine-based monomers are used as raw materials and synthesized through Mannich condensation reaction under certain conditions to synthesize a series of polymers with relatively small molecular weights. Such polymers can be ring-opened and polymerized under the action of heating or catalysts.
[0034] This implementation mode has the following special effects:
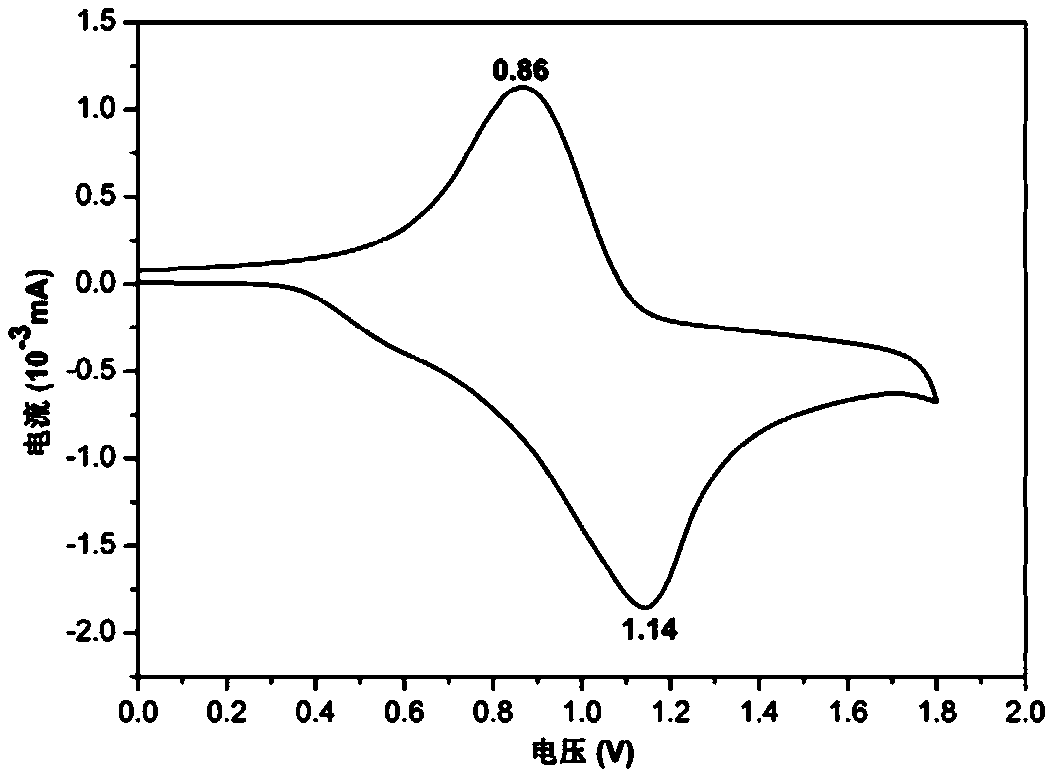
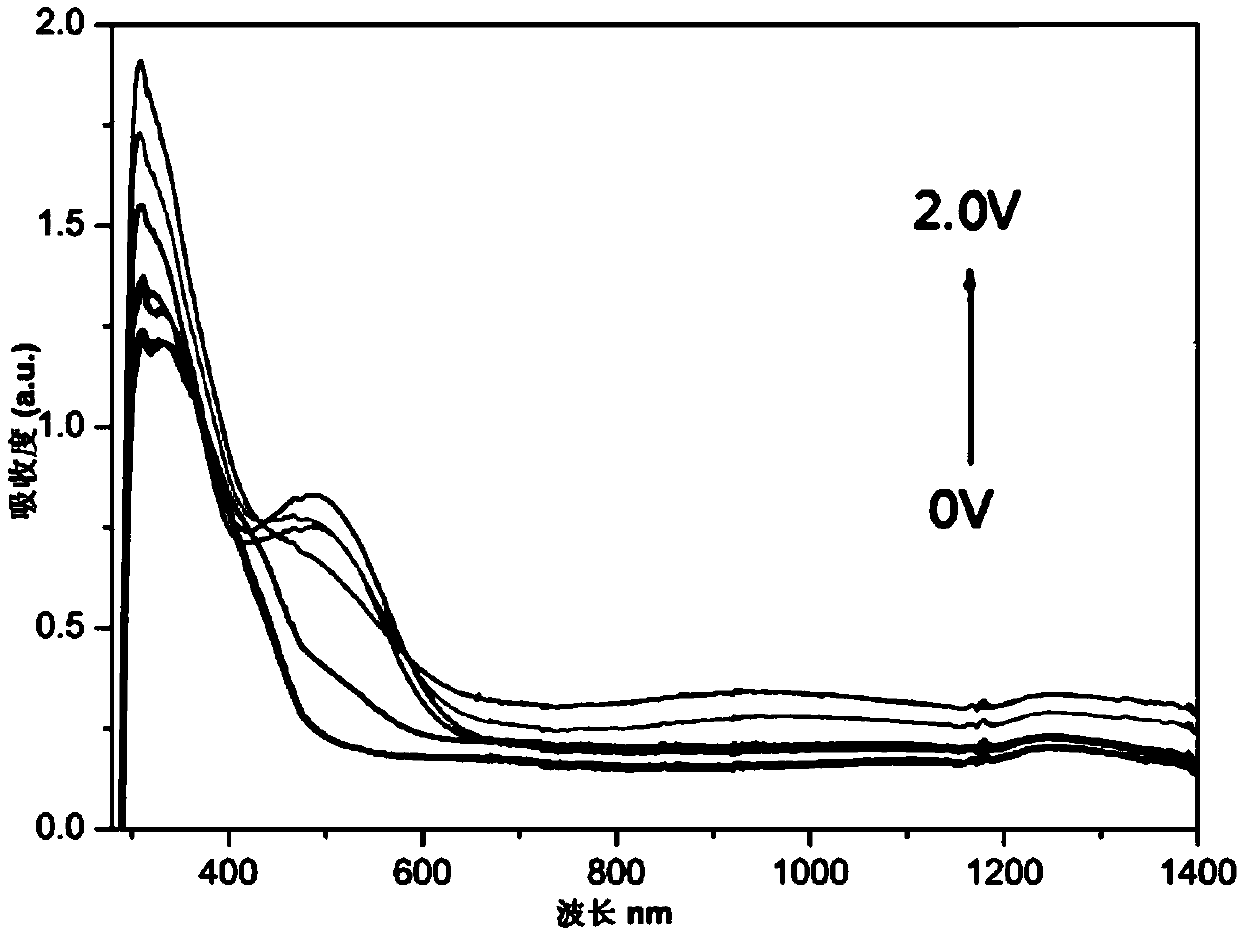
[0035] 1. In this embodiment, the benzoxazine structure is introduced into...
specific Embodiment approach 2
[0040] Specific embodiment two: the preparation method of a kind of multifunctional triarylamino-containing oxazine polymer of this embodiment is:
[0041] 1. N,N'-bis(4-aminophenyl)-N,N'-di-2-naphthyl-1,4-biphenyldiamine
[0042] in N 2 Atmosphere, set N 4 ,N 4 - Di-naphthalene-2-yl-biphenyl-4,4-diamine monomer, sodium hydride and anhydrous N, N-dimethylformamide are placed in a three-necked flask, and stirred at 1 to 2 drops per second Add p-fluoronitrobenzene at the dropping speed, heat up to 114-115°C, carry out constant temperature reaction, and then cool down; put the reaction product in water at 24-25°C until the crude product precipitates, then filter out the crude product, and use 99-100°C The crude product was washed with water for 2 to 3 times, the obtained crude product was dried in a vacuum drying oven, and then recrystallized with ethanol, and the crystallized product was filtered out after recrystallized, and the crystallized product was vacuum-dried to obtai...
specific Embodiment approach 3
[0047] Specific embodiment three: the difference between this embodiment and specific embodiment two is that the powder M2 in step one ① is N, N'-bis(4-nitrophenyl)-N, N'-two-2-naphthyl -1,4-Benphenyldiamine. Others are the same as in the second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com