Human immunodeficiency virus type 1 (HIV-1) nucleic acid quantitative detection kit
A human immunodeficiency, detection kit technology, applied in the biological field, can solve the problems of antibody decline, immune cross-reaction cycle, low sensitivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
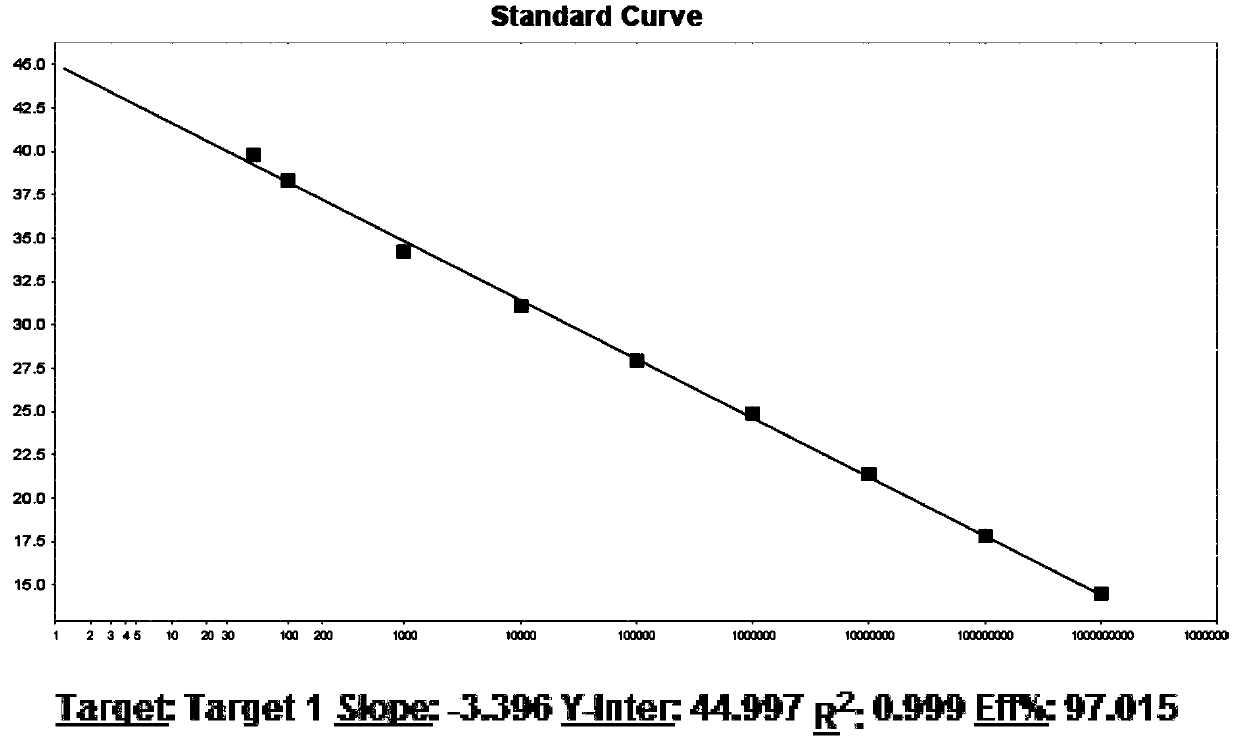
[0061] On the basis of comparing the nucleic acid sequences of HIV-1 known on GENBANK and the international HIV database, the specific conserved regions of the nucleic acid sequences of various subtypes were searched for, and the LTR regions and the distant LTR regions were selected. Two target nucleotide probes and corresponding primers were designed on the two conserved regions of the gene in the GAG region. These primer probes are used in RT-PCR reaction enzyme systems containing thermostable DNA polymerase, reverse transcriptase, high-quality deoxyribonucleoside triphosphates (dNTPs) and Mg 2+ In the RT-PCR reaction solution with equal components, the circular amplification of nucleic acid in vitro is realized by a fluorescent PCR instrument.
[0062] According to the embodiment of the present invention, the main components of the kit involved in the present invention include: HIV-1 reaction solution A, HIV-1 reaction solution B, HIV-1 quantitative reference substance, n...
Embodiment 1
[0084] Embodiment 1: human immunodeficiency virus type 1 (HIV-1) nucleic acid quantitative detection kit (PCR-fluorescent probe method)
[0085] This kit uses fluorescent quantitative PCR method to quantitatively detect human immunodeficiency virus type 1 nucleic acid (HIV-1) RNA in serum or plasma samples, and is suitable for auxiliary diagnosis of human immunodeficiency virus type 1 infection and human immunodeficiency virus Monitoring the efficacy of drug therapy in people with type 1 infection.
[0086] Reagents included in the kit:
[0087]
[0088] Specific primer probe sequences are as above.
[0089] Inspection program:
[0090] 1. Sample processing and nucleic acid extraction (sample processing area)
[0091] The negative quality control, positive quality control, and quantitative reference in this kit are all involved in the extraction, and are used for monitoring the environment and quality control of PCR detection reagents. The internal standard solution in ...
Embodiment 2
[0128] Embodiment 2: Sensitivity detection experiment
[0129] Sensitivity reference samples come from a nominal concentration of 5×10 5 The national reference product of IU / ml (China Food and Drug Control Institute), with HIV-1 negative plasma diluted national reference product to 20IU / ml as the sensitivity reference product, totally 20 parts. Nucleic acid extraction was performed simultaneously with 20 sensitivity reference products, negative quality control products, HIV-1 strong positive quality control products, HIV-1 critical positive quality control products and 4 positive quantitative reference products.
[0130] The experiment was repeated 3 times in parallel and all obtained the same detection results, showing that the primers of the present invention have good specificity, the system has good amplification efficiency, and the sensitivity detection is good. The sensitivity of the kit of the present invention is 20IU / ml and repeated 20 times. Test, 3 The positive det...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com