Combination therapy for COPD
A technology for inhalation therapy and use, applied in the field of prevention and treatment of respiratory disorders), which can solve problems such as short storage period and instability of aerosol solution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0160] Embodiment 1. Preparation of fixed ternary combination aerosol solution preparation
[0161] Compositions of formoterol fumarate dihydrate (FF), beclomethasone dipropionate (BDP) and glycopyrronium bromide (GB) were prepared as shown in Table 2 and packaged on FEP-coated In aluminum cans equipped with EPDM valves with a 63 μl metering chamber.
[0162] Table 2. Composition of an aerosol solution composition of a fixed ternary combination of formoterol fumarate (FF) dihydrate, glycopyrronium bromide (GB) and beclomethasone dipropionate (BDP). Content % w / w refers to the content percentage of the weight of each component relative to the total weight of the composition.
[0163]
Embodiment 2
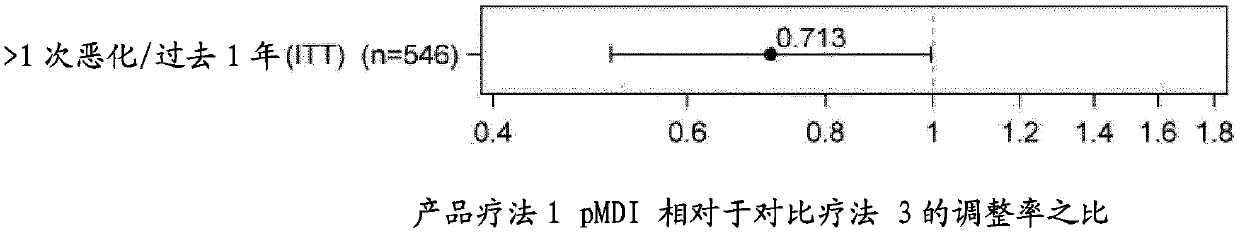
[0164] Example 2. Comparison of the administration of the product (1) according to the invention with respect to the comparative therapy (2) and the comparative therapy (3)
[0165] Product therapy (1): It is the fixed-dose pMDI solution formulation of Example 1, consisting of beclomethasone dipropionate (BDP) 100 μg / actuation, formoterol fumarate (FF) dihydrate 6 μg / actuation Composed of a ternary combination with glycopyrronium bromide (GB) 12.5 μg / actuation, administered in 2 actuations twice daily (b.i.d.).
[0166] Comparator therapy (2): It is a DPI formulation of Tiotropium bromide (Tio) 18 μg / actuation (Spiriva Handihaler), administered in 1 actuation once a day.
[0167] Comparator therapy (3): It is a fixed-dose pMDI solution formulation of a binary combination of BDP 100 μg / actuation and FF 6 μg / actuation (administered in 2 actuations b.i.d.) + tiotropium Immediate triple combination of DPI formulation (administered in 1 actuation once daily) at 18 μg / actuation.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com