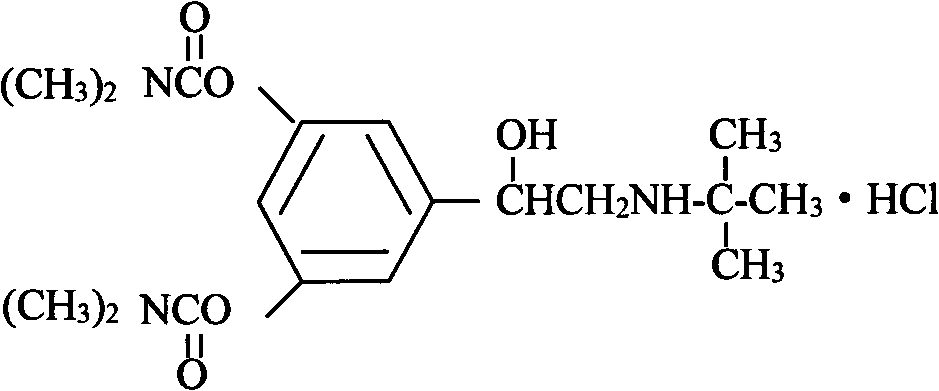
Bambuterol hydrochloride and roflumilast compound preparation and its preparation method
A technology of bambuterol hydrochloride and roflumilast, which is applied in the direction of pill delivery, active ingredients of esters, active ingredients of heterocyclic compounds, etc., can solve problems such as inapplicability, and achieve convenience in taking, good taste, and simple preparation process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0030] Babuterol hydrochloride fast-release layer and roflumilast slow-release layer compound double-layer tablet, including main drug and auxiliary materials, is characterized in that it is formulated according to the following weight percentage: main drug: 2.625%, auxiliary materials: 97.375% .
[0031] Immediate-release tablet formulation:
[0032]
[0033] Extended-release tablet formulation:
[0034]
[0035]
[0036] Among them, corn starch, lactose, and microcrystalline cellulose are fillers; hydroxypropyl methylcellulose k4M and hydroxypropyl methylcellulose k15M are binders; cross-linked polyvinylpyrrolidone is a disintegrant; gelatin, talc The powder is effervescent; distilled water and magnesium stearate are lubricants.
[0037] The preparation method of the compound double-layer tablet of bambuterol hydrochloride quick-release layer and roflumilast slow-release layer of the present invention comprises raw material crushing, weighing, mixing uniformly, an...
example 2
[0042] Babuterol hydrochloride fast-release layer and roflumilast sustained-release layer compound double-layer tablet, including main drug and auxiliary materials, is characterized in that it is formulated according to the following weight percentages: main drug: 5.25%, auxiliary materials: 94.75% .
[0043] Immediate-release tablet formulation:
[0044]
[0045]
[0046] Extended-release tablet formulation:
[0047]
[0048] Among them, corn starch, lactose, and microcrystalline cellulose are fillers; hydroxypropyl methylcellulose k4M and hydroxypropyl methylcellulose k15M are binders; cross-linked polyvinylpyrrolidone is a disintegrant; gelatin, talc The powder is effervescent; distilled water and magnesium stearate are lubricants.
[0049] The preparation method of the compound double-layer tablet of bambuterol hydrochloride quick-release layer and roflumilast slow-release layer of the present invention comprises raw material crushing, weighing, mixing uniformly, ...
Embodiment 3
[0054] The compound syrup of bambuterol hydrochloride and roflumilast comprises main ingredients and auxiliary materials, and is characterized in that it is formulated according to the following weight percentages: main ingredients: 0.0512%, auxiliary materials: 99.9488%. Compound syrup of the present invention is formulated from the raw materials of following weight ratio:
[0055]
[0056] Among them, liquid glucose and sucrose are sweet additives; vitamin C is an antioxidant; sodium metabisulfite is a preservative; edetate disodium is a complexing agent; purified water is a diluent.
[0057] The preparation method of bambuterol hydrochloride and roflumilast compound syrup of the present invention comprises the steps of pulverizing raw materials, weighing, mixing, stirring, filtering and sterilizing, and is characterized in that the specific process steps are as follows:
[0058] Step 1: Pulverize bambuterol hydrochloride, roflumilast and the above-mentioned various excip...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com