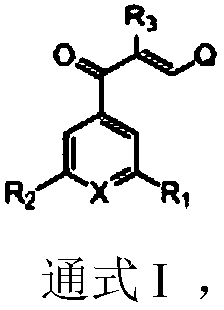
Pyridine substituted chalcone type compound or medicinal salt thereof and preparation method and application thereof
A technology for chalcones and compounds, which is applied in the field of pyridine-substituted chalcones or their pharmaceutically acceptable salts and their preparations, which can solve the problems of large toxic and side effects, poor water solubility, and difficult synthesis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104]
[0105] (E)-1-(2,6-dimethoxypyridin-4-yl)-3-(4-methoxyphenyl)-2-methylpropane-2-en-1-one
[0106] (a) Dissolve 2,6-dichloroisonicotinic acid (10g, 52mmol) in anhydrous THF, under nitrogen protection, drop into 2M ethylmagnesium bromide (80ml, 160mmol) under ice-bath conditions, react at room temperature for 2h, then add water Dilute, extract with dichloromethane (50mL×3), combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, concentrate and column chromatography (PE / EA 4:1) to give 1-(2,6-dichloropyridine -4-yl)propan-1-one 9g, yield 85%;
[0107] (b) Dissolve 1-(2,6-lutidine-4-yl)propan-1-one (6g, 29.4mmol) in toluene, add DBU (12.43ml, 88.2mmol), 2-bromoethanol ( 10.42ml, 147mmol), react overnight at 80°C. Spin dry toluene, dilute with water, extract with dichloromethane (20mL×3), combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, concentrate and column chromatography (PE / EA 40:1) to give...
Embodiment 2
[0112]
[0113] (E)-3-(3,4-dimethoxyphenyl)-1-(2,6-dimethoxypyridin-4-yl)-2-methylpropane-2-en-1-one
[0114] According to the operation of Example 1(d), 100 mg of a yellow solid was obtained with a yield of 72%;
[0115] 1 H NMR (300MHz, CDCl 3 )δ7.21(s, 1H), 7.14-7.03(m, 3H), 6.45(s, 2H), 3.93(s, 6H), 3.88(s, 6H), 2.11(s, 3H); 13 C NMR (75MHz, CDCl 3 )δ196.33, 165.45, 150.34, 149.26, 149.11, 138.82, 136.39, 129.75, 124.08, 112.72, 111.80, 101.84, 55.98, 55.89, 53.80, 16.41; ESI-MS m / z: 343.1 C for calc 19 h 22 NO 5 [M+H] + 344.1.
Embodiment 3
[0117]
[0118] (E)-1-(2,6-dimethoxypyridin-4-yl)-2-methyl-3-(3,4,5-trimethoxyphenyl)-propane-2-ene-1 -ketone
[0119] According to the operation of Example 1(d), 82 mg of yellow solid was obtained, and the yield was 70%;
[0120] 1 H NMR (300MHz, CDCl 3)δ7.20(s, 1H), 6.64(s, 2H), 6.47(s, 2H), 3.97(s, 6H), 3.89(s, 3H), 3.88(s, 6H), 2.25(s, 3H ); 13 C NMR (75MHz, CDCl 3 )δ196.33, 165.44, 153.30, 149.27, 139.51, 138.49, 136.07, 130.20, 107.70, 101.85, 60.83, 56.20, 53.79, 16.33; ESI-MS m / z: 373.1 calcd for C 20 h 24 NO 6 [M+H] + 374.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com