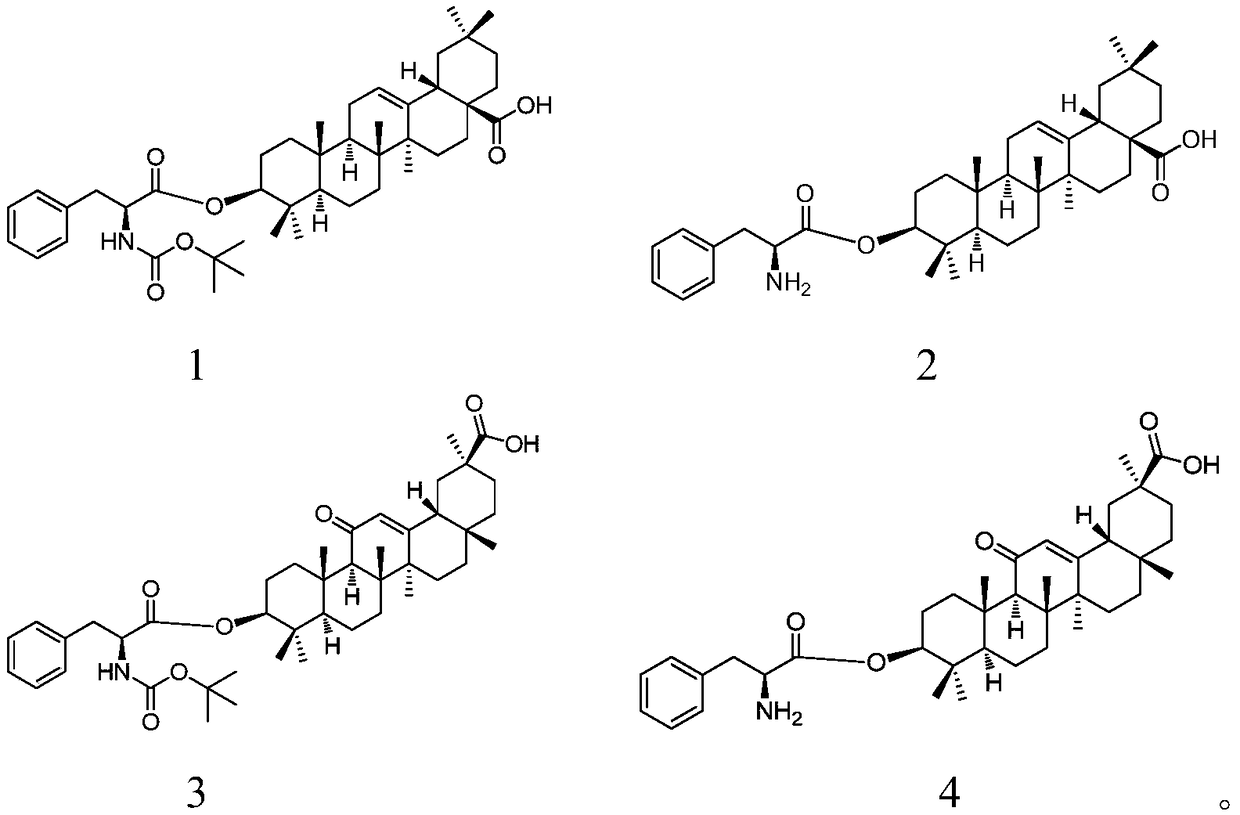
3-(L-phenylalanine)-pentacyclic triterpene derivatives as well as synthetic method and application thereof
A technology of phenylalanine and synthesis method, applied in the direction of drug combination, medical preparations containing active ingredients, pharmaceutical formulas, etc., to achieve good proliferation inhibitory activity and good medicinal value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
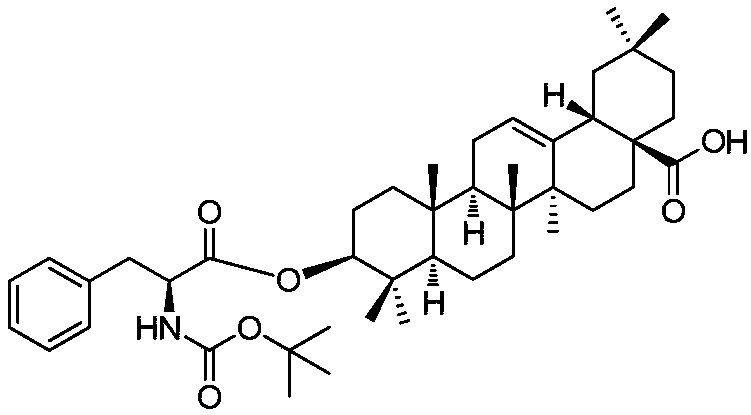
[0025] Embodiment 1: the synthesis of compound 1
[0026]
[0027] Get oleanolic acid (2.00g, 4.38mmol) and Boc-L-phenylalanine (1.74g, 6.57mmol) and dissolve in CH 2 Cl 2 (50mL), DCC (1.80g, 8.77mmol) and DMAP (0.10g, 0.87mmol) were added successively, stirred and reacted for 24h at room temperature, the obtained reaction material was filtered, the filter cake was washed with dichloromethane (20mL), and the filtrate was collected , spin-dried under reduced pressure, the obtained residue was dispersed in EtOAc (200 mL), washed with water and saturated brine successively, dried over anhydrous sodium sulfate, filtered, and the filtrate was spin-dried to obtain a crude product. The crude product was separated by silica gel column chromatography (eluent: V 石油醚 :V 乙酸乙酯 =8:1-5:1), to obtain compound 1 (1.98g, 64.0%, white solid).
[0028] Yield: 1.98g, 64.0%, white solid; R f =0.72(Petroleum ether:EtOAc=2:1).m.p.116-118℃. 1 H NMR (500MHz, CDCl 3 ):δ0.73(s,3H,CH 3 ),0.79(s...
Embodiment 2
[0029] Embodiment 2: the synthesis of compound 1
[0030] Take oleanolic acid (2.00g, 4.38mmol) and Boc-L-phenylalanine (1.74g, 6.57mmol) and dissolve in MeOH (50mL), add DCC (1.80g, 8.77mmol), DMAP (0.10 g, 0.87mmol), stirring and reacting at room temperature for 10h, the resulting reaction material was filtered, the filter cake was washed with MeOH (20mL), the filtrate was collected, spin-dried under reduced pressure, and the resulting residue was separated by silica gel column chromatography (eluent: V 石油醚 :V 乙酸乙酯 =8:1-5:1), a white solid (1.21 g, 40.0%) was obtained.
[0031] The obtained product was characterized by H NMR spectrum and identified as compound 1.
Embodiment 3
[0032] Embodiment 3: the synthesis of compound 2
[0033]
[0034] Take compound 1 (0.20g, 0.28mmol) and dissolve in CH2 Cl 2 (15mL), add TFA (0.6mL, 0.2mL / 1h), stir the reaction at room temperature for 3h, add saturated aqueous sodium bicarbonate solution dropwise to the material obtained in the reaction until no bubbles are generated, and use CH 2 Cl 2 (3×50 mL), combined the organic layers, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and the filtrate was spin-dried to obtain the crude product. The crude product was separated by silica gel column chromatography (eluent: V 石油醚 :V 乙酸乙酯 =3:1-1:1), to obtain compound 2 (0.11 g, 64.0%, white solid).
[0035] Yield: 0.11g, 64.0%, white solid; R f =0.31(Petroleum ether:EtOAc=1:1).m.p.144-146℃. 1 H NMR (400MHz, CDCl 3 ):δ0.74,0.80,0.82,0.89,0.921,0.926,1.12(7s,each 3H,7×CH 3 ),0.84-2.00(m,22H),2.79-2.86(m,2H,H-18,phCH),3.14(dd,J=5.4,13.6Hz,1H,phCH),3.74-3.77(m,1H, NCHCOO), 4.50-4.54(m, 1H,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com