Omega-transaminase mutant capable of catalyzing sitafloxacin five-membered key intermediate
A mutant, transaminase technology, applied in the field of genetic engineering and enzyme engineering, can solve the problems of inability to apply ω-transaminase, reduce development and application, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Preparation and construction of ω-transaminase site-directed mutants
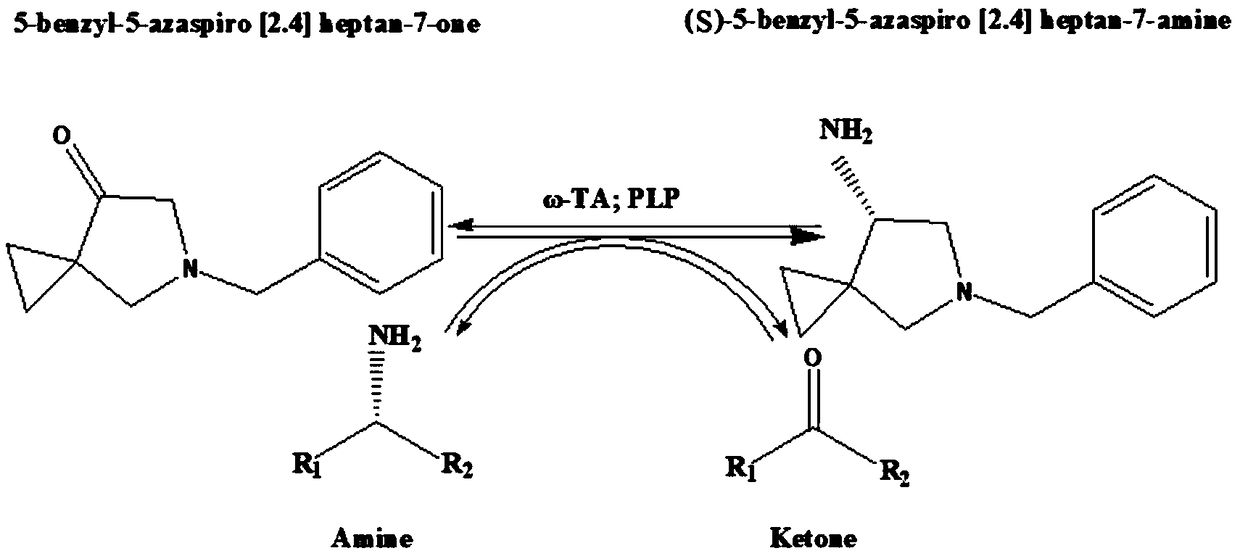
[0028] One site-directed mutant Y32L / S190A / L212M / I215M of ω-transaminase derived from Bacillus pumilus W3:
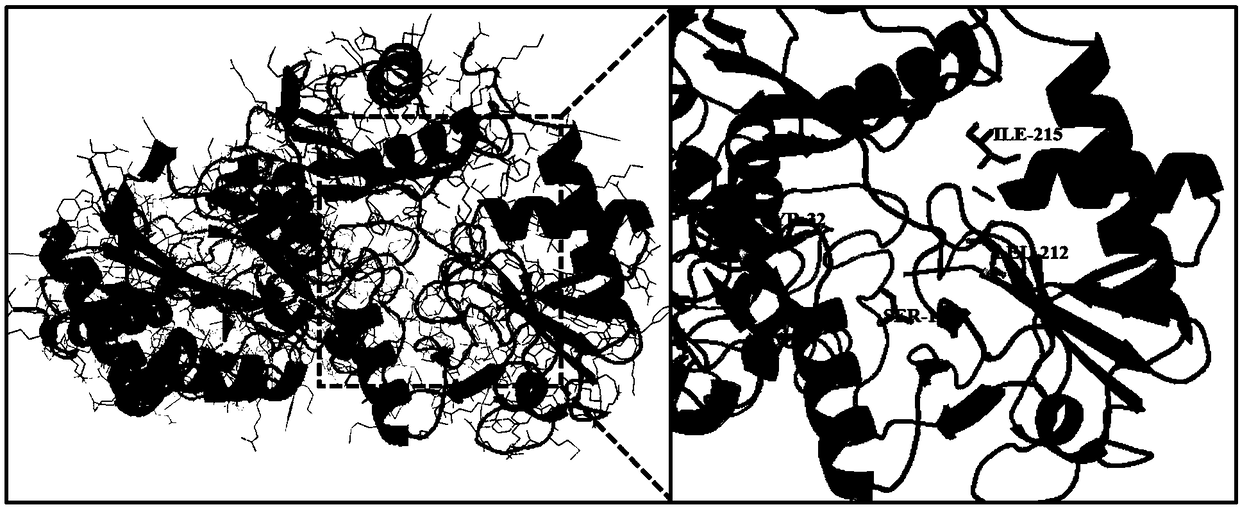
[0029] In the present invention, the most similar thermophilic archaea transaminase crystal structure (PDB ID: 5E25) is used as a template to construct the three-dimensional simulation structure of Bacillus pumilus W3 ω-transaminase (ω-BPAT) through the Swiss-Model online server ( figure 1 ). Through amino acid primary sequence alignment, it was found that the similarity between thermophilic archaea aminotransferase and ω-BPAT reached 51.21%, which was in line with the parameters of homology modeling. Therefore, it can be considered that ω-BPAT has a similar three-dimensional structure to thermophilic archaea aminotransferase. According to the results of software analysis and prediction, mutant Y32L / S190A / L212M / I215M was constructed by PCR-mediated site-directed mutagenesis.
[00...
Embodiment 2
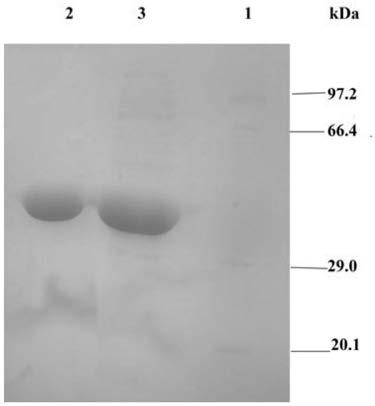
[0041] Example 2: Expression and purification of native ω-transaminase and site-directed mutants thereof
[0042] Pick the positive single clones transferred into the expression host Escherichia coli BL21 (DE3) and grow them in LB liquid medium (containing 30 μg / mL ampicillin) for 8-10 hours, and transfer the seed fermentation broth to LB liquid medium ( containing 30 μg / mL ampicillin); Escherichia coli was cultured on a shaker at 37°C for 2 hours until OD 600 = about 0.6, the mutant Y32L / S190A / L212M / I215M recombinant strain was added with 0.05mM final concentration of IPTG to induce extracellular expression, and continued to culture and ferment on a shaker at 15°C for 24h, then centrifuged the fermentation broth at 4°C and 8000g for 10min to remove Bacteria, and the centrifuged fermentation supernatant was collected. Slowly add 60% (NH 4 ) 2 SO 4 , placed at 4°C for salting out overnight. Centrifuge at 10,000 g for 20 min at 4°C to collect the precipitate. After redisso...
Embodiment 3
[0043] Embodiment 3: enzyme activity analysis method
[0044] The determination method of ω-transaminase activity refers to Gao, S. (Gao, S., Su, Y., Zhao, L., Li, G., Zheng, G., 2017.Characterization of a(R)-selective amine transaminase from Fusariumoxysporum. Process. Biochem. 63, 130-136.).
[0045] Take an appropriate amount of bacterial supernatant (or purified and diluted enzyme solution), add 500 μL sodium dihydrogen phosphate / disodium hydrogen phosphate buffer (100 mM, pH7.0), which contains 20 mM (R)-α-phenethylamine ( or (S)-α-phenethylamine), 20mM sodium pyruvate, 0.1mM pyridoxal 5'-phosphate (PLP), mix well, react at 45°C for 15min respectively, and then add the same amount of ethyl acetate to terminate the reaction. The absorbance of the solution at 254 nm was measured before and after the reaction.
[0046] Under the above conditions, the amount of enzyme required to catalyze 1 μmol of related ketones in 1 minute is defined as one enzyme activity unit (U / ml). ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com