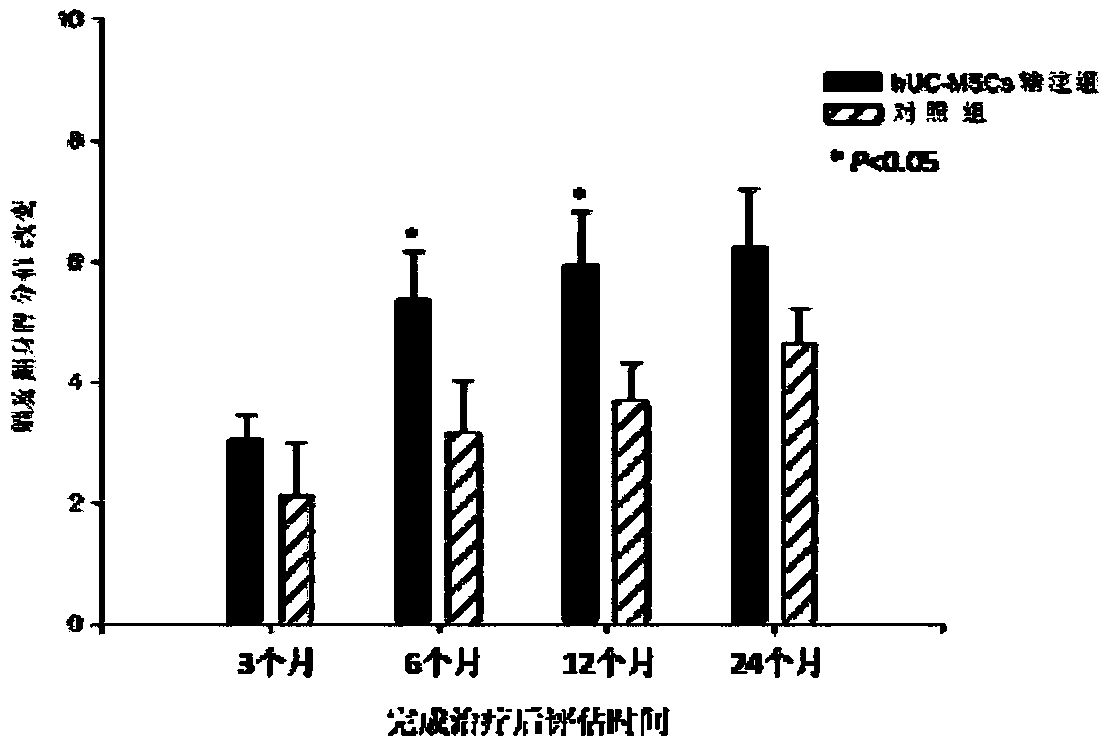
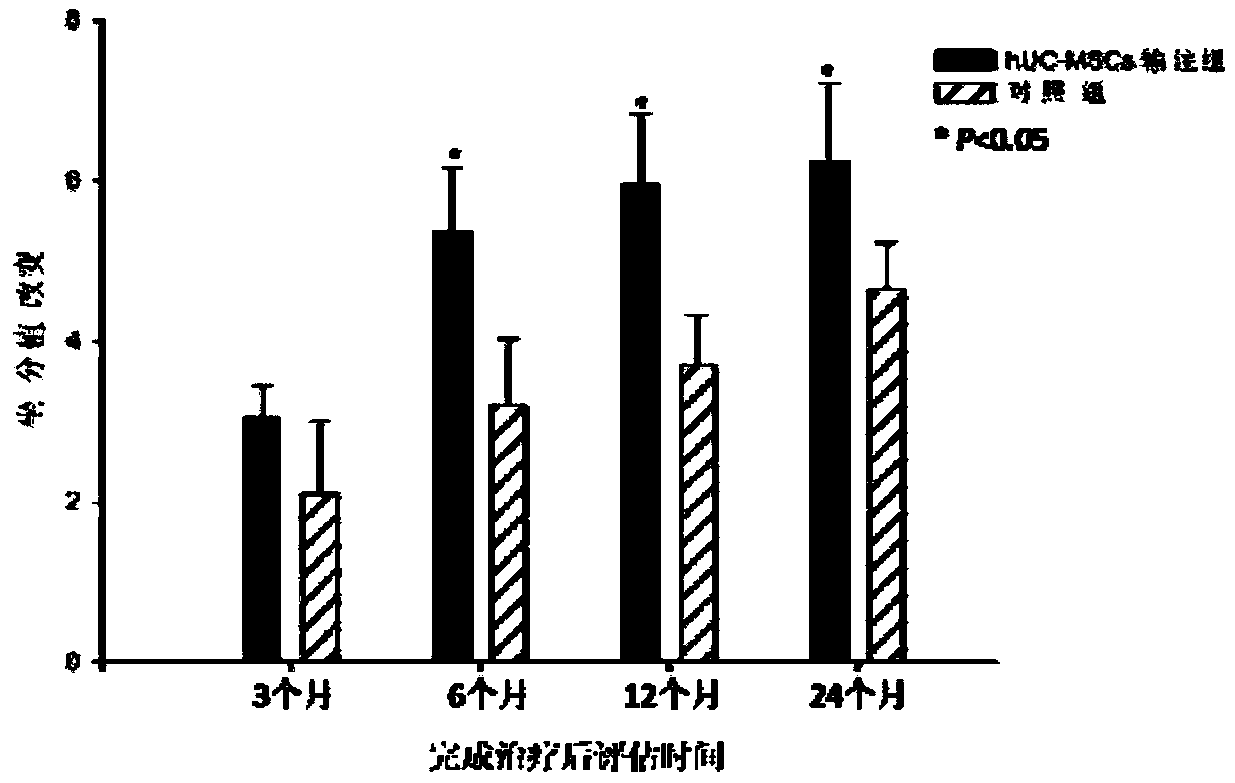
Application of human umbilical cord mesenchymal stem cells in preparing drugs for treating cerebral palsy and culture method
A technology of stem cells and culture method, which is applied to the application and culture of human umbilical cord mesenchymal stem cells in the preparation of cerebral palsy drugs, can solve problems such as little effect, and achieve the effects of less trauma, good patient compliance, and large cell volume
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] This embodiment provides the application and culture method of human umbilical cord mesenchymal stem cells in the preparation of cerebral palsy medicine. The specific treatment method includes the following steps:
[0039] (1) Take the human umbilical cord ligated after delivery of the fetus of a puerpera without infectious diseases, wash it with a sterilized PBS solution, place it in a PBS solution containing 900 U / mL gentamicin, and store it at a constant temperature at 2°C;
[0040] (2) the Wharton's colloid obtained after the separation of the human umbilical cord is cut into 1-4mm 3 The tissue homogenate block was added to the serum-free medium of human umbilical cord mesenchymal stem cells for culturing under the conditions of 3% carbon dioxide, constant temperature of 36°C, and humidity of 90%, to obtain cell clones;
[0041] (3) When the cell cloning group grows to 70% of the area of the culture bottle wall, it is digested with 0.2% trypsin for 1 min, and the ...
Embodiment 2
[0048] This embodiment provides the application and culture method of human umbilical cord mesenchymal stem cells in the preparation of cerebral palsy medicine. The specific treatment method includes the following steps:
[0049] (1) Take the human umbilical cord ligated after delivery of the fetus of a puerpera without infectious diseases, wash it with a sterilized PBS solution, place it in a PBS solution containing 1100 U / mL gentamicin, and store it at a constant temperature at 8°C;
[0050] (2) the Wharton's colloid obtained after the separation of the human umbilical cord is cut into 4mm 3 The tissue homogenate was added to the serum-free medium of human umbilical cord mesenchymal stem cells for culture, the culture conditions were 7% carbon dioxide, 38°C constant temperature, and 100% humidity to obtain cell clones;
[0051] (3) When the cell cloning group grows to an area accounting for 90% of the wall area of the culture bottle, it is digested with 0.3% trypsin for 3 ...
Embodiment 3
[0058] This embodiment provides the application and culture method of human umbilical cord mesenchymal stem cells in the preparation of cerebral palsy medicine. The specific treatment method includes the following steps:
[0059] (1) Take the human umbilical cord ligated after delivery of the fetus of a puerpera without infectious diseases, wash it with a sterilized PBS solution, place it in a PBS solution containing 1000 U / mL gentamicin, and store it at a constant temperature at 6°C;
[0060] (2) the Wharton's colloid obtained after the separation of the human umbilical cord is cut into 2mm 3 The tissue homogenate was added to the serum-free medium of human umbilical cord mesenchymal stem cells for culture, the culture conditions were 5% carbon dioxide, 37°C constant temperature, and 95% humidity to obtain cell clones;
[0061] (3) When the cell cloning group grows to 80% of the area of the culture bottle wall, it is digested with 0.25% trypsin for 2 minutes, and the volume...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com