Alkadiene removal method for FCC gasoline light fractions
A technology for separating and removing diolefins and gasoline is applied in the field of selective hydrotreating and removing diolefins in the light ends of catalytically cracked gasoline to achieve the effect of reducing the surface coking process, strong adaptability of gasoline and improving arsenic resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1. Preparation of lanthanum magnesium aluminate LaMgAl 11 o 19 The process is as follows:
[0023] Take 7.8 grams of basic magnesium carbonate, 25 grams of lanthanum carbonate and 70.5 grams of aluminum hydroxide, mix well, add deionized water to form a highly dispersed suspension, spray dry, dry at 140 ° C for 5 hours, and place in an air atmosphere at 1300 ℃ for 4 hours, and then ball milled for 18 hours to obtain magnesium lanthanum aluminate powder. Add water to magnesium lanthanum aluminate powder, and obtain highly dispersed magnesium lanthanum aluminate slurry after ultrasonic vibration.
[0024] 2. Preparation of silica-alumina carrier
[0025] Take 170g of pseudo-boehmite and 30g of scallop powder, mix evenly, add dilute nitric acid, then add 32g of sodium polyacrylate nitric acid solution, and mix evenly, then add magnesium lanthanum aluminate slurry, mix evenly, and obtain aluminum-containing and aluminum-containing The mixture (1) of magnesium lanthanum ...
Embodiment 2
[0029] The preparation of magnesium lanthanum aluminate is the same as in Example 1, the preparation of the silica-alumina carrier is the same as in Example 1, and 68% of pseudo-boehmite is mixed evenly with the scallop powder, and the addition of pore-enlarging agent 1 sodium polyacid accounts for 19% of the mass of alumina was added, and the amount of pore-enlarging agent 2 methylcellulose accounted for 4% of the mass of alumina and silica added. The silica-alumina carrier contains 7.2 wt% of silica and 6.6 wt% of magnesium lanthanum aluminate.
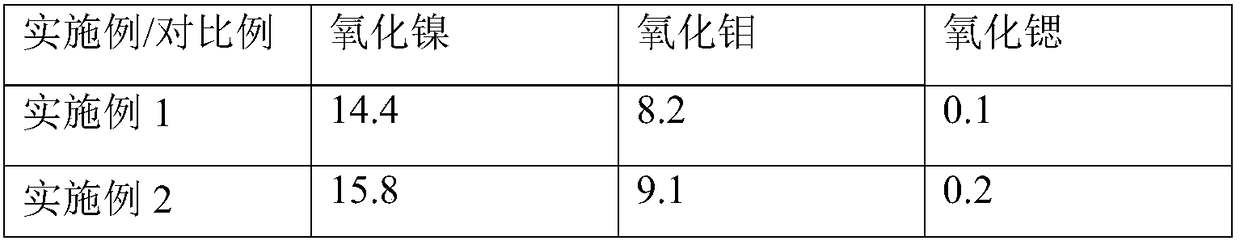
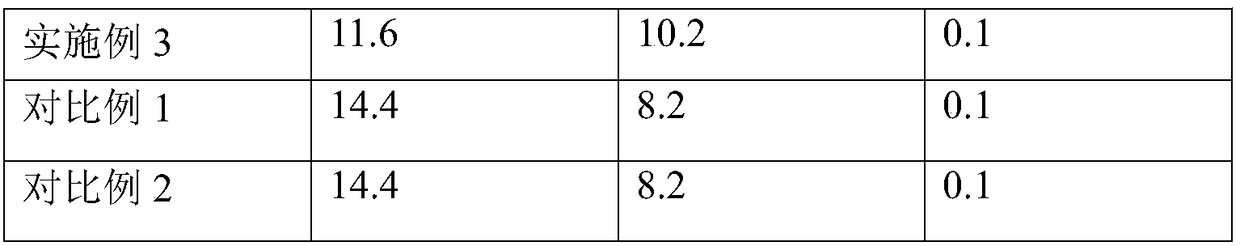
[0030] The preparation method of the catalyst is the same as in Example 1, and the composition of the catalyst is shown in Table 1.
Embodiment 3
[0032] Preparation of nickel-containing magnesium lanthanum aluminate: get 7.8 grams of basic magnesium carbonate, 25 grams of lanthanum carbonate and 70.5 grams of aluminum hydroxide, mix well, add deionized water to form a highly dispersed suspension, then add 8 grams of nickel nitrate, through Spray drying, drying at 140° C. for 5 hours, calcination at 1300° C. for 4 hours in an air atmosphere, and ball milling for 18 hours to obtain nickel-containing magnesium lanthanum aluminate powder. The nickel-containing magnesium-lanthanum aluminate powder is added with water, and the highly dispersed nickel-containing magnesium-lanthanum aluminate slurry is obtained after ultrasonic vibration.
[0033] The preparation of the silica-alumina carrier is the same as in Example 1, except that the nickel-containing magnesium-lanthanum aluminate slurry is added, and 72% of the pseudo-boehmite is mixed evenly with the celadon powder, and the amount of the pore-enlarging agent 1 polyacrylate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com