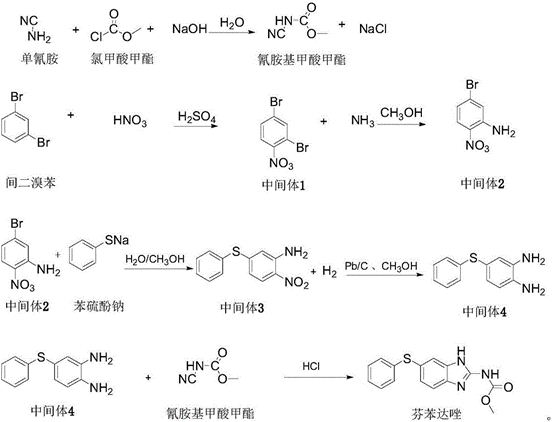
A kind of preparation method of fenbendazole
A technology of fenbendazole and preparation process, applied in the field of preparation of fenbendazole, can solve the problem of high production cost, achieve the effects of reduced production cost, mild reaction conditions, and avoidance of the use of thiophenol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] (1) Preparation of methyl cyanamide formate aqueous solution
[0017] 44.0g of cyanamide aqueous solution was cooled to 0-5°C, and 10.93g of methyl chloroformate and 16.03g of sodium hydroxide solution (30%) were added dropwise under stirring. The material was dripped at the same time or the methyl chloroformate was dripped slightly earlier than the sodium hydroxide solution. After the dropwise addition of both materials, the mixture was stirred at 0-5° C. for 2 hours to obtain 70.91 g of an aqueous methyl cyanamide formate solution with a concentration of 14.0%, which was stored at about 0° C. for low temperature for later use.
[0018] (2) Intermediate 1 (2,4-dibromonitrobenzene)
[0019] Dissolve 24.5g m-dibromobenzene into 49.0g concentrated sulfuric acid (98%) at room temperature, cool to 0-5°C, add 9.80g nitric acid (68%) dropwise, and control the temperature at 0-5°C during the dropping process. After the addition was complete, stir at 5-10°C for 2 hours. Afte...
Embodiment 2
[0029] (1) Preparation of methyl cyanamide formate aqueous solution
[0030] 220.0g of cyanamide aqueous solution was cooled to 0-5°C, and 54.65g of methyl chloroformate and 80.15g of sodium hydroxide solution (30%) were added dropwise under stirring. The material was dripped at the same time or the methyl chloroformate was dripped slightly earlier than the sodium hydroxide solution. After the dropwise addition of both materials, the mixture was stirred at 0-5° C. for 2 hours to obtain 355.0 g of an aqueous methyl cyanamide formate solution with a concentration of 14.0%, which was stored at about 0° C. for low temperature for later use.
[0031] (2) Intermediate 1 (2,4-dibromonitrobenzene)
[0032] Dissolve 122.5g m-dibromobenzene into 245.0g concentrated sulfuric acid (98%) at room temperature, cool to 0-5°C, add 49.0g nitric acid (68%) dropwise, control the temperature during the dropwise addition at 0-5°C, dropwise After the addition was complete, stir at 5-10°C for 2 hou...
Embodiment 3
[0042] (1) Preparation of methyl cyanamide formate aqueous solution
[0043] 440.0g of cyanamide aqueous solution was cooled to 0-5°C, and 109.3g of methyl chloroformate and 160.3g of sodium hydroxide solution (30%) were added dropwise under stirring. The material was dripped at the same time or the methyl chloroformate was dripped slightly earlier than the sodium hydroxide solution. After the dropwise addition of both materials, the mixture was stirred at 0-5° C. for 2 hours to obtain 708.0 g of an aqueous methyl cyanamide formate solution with a concentration of 14.0%, which was stored at about 0° C. for low temperature for later use.
[0044] (2) Intermediate 1 (2,4-dibromonitrobenzene)
[0045] Dissolve 245.0g m-dibromobenzene into 490.0g concentrated sulfuric acid (98%) at room temperature, cool to 0-5°C, add 98.0g nitric acid (68%) dropwise, control the temperature in the dropping process at 0-5°C, dropwise After the addition was complete, stir at 5-10°C for 2 hours. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com