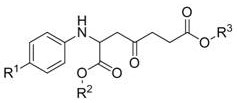
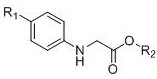
A kind of synthetic method of α-amino-γ-carbonylpimelate compound
A technology of carbonylpimelic acid ester and synthesis method, which is applied in the preparation of organic compounds, chemical instruments and methods, and preparation of cyanide reactions, etc., and can solve problems such as difficult synthesis or acquisition of reaction substrates, negative environmental impacts, and increased costs , to achieve the effects of fast reaction rate, less environmental pollution and mild synthesis conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Synthesis of 7-benzyl 1-methyl-4-oxo-2-(p-tolylamino)pimelate
[0026]
[0027] In a 25 ml round-bottomed flask, add magnetons, add 2-(p-tolylamino)methyl acetate (0.3 mmol), acetonitrile (6 ml), CuCl 2 (20 mol%), H 2 SO 4 (30 mol %) , stirred at room temperature for 2 hours, washed and extracted, separated by column chromatography; then added BnBr (3.2eq), K 2 CO 3 (2.4eq), DMF, reacted for 1 hour, washed to remove DMF, and separated by column chromatography (silica gel: 200~300 mesh, eluent volume ratio: petroleum ether: acetone = 3:1), to obtain a light yellow oily liquid The pure product is 7-benzyl 1-methyl-4-oxo-2-(p-tolylamino)pimelate. The yield was 50%.
[0028] The NMR data of this compound are as follows: 1 H NMR (600 MHz, CDCl 3 ) δ 7.37 – 7.34 (m, 2H),7.33 (d, J = 2.2 Hz, 2H), 7.32 (d, J = 3.1 Hz, 1H), 6.98 (d, J = 8.1 Hz, 2H),6.56 (d , J = 8.4 Hz, 2H), 5.10 (s, 2H), 4.42 (d, J = 5.3 Hz, 1H), 4.23 (s,1H), 3.70 (s, 3H), 3.00 (d, J = 5.5...
Embodiment 2
[0029] Embodiment 2: the synthesis of 4-oxo-2-((4-phenoxyphenyl) amino) dimethyl pimelate
[0030]
[0031]In a 25 ml round-bottomed flask, add magnetons, add 2-((4-phenoxyphenyl)amino)methyl acetate (0.3 mmol), acetonitrile (6 ml), CuCl 2 (20 mol%), H 2 SO 4 (30 mol %) , stirred at room temperature for 2 hours, washed and extracted, separated by column chromatography; then added CH 3 I (3.2eq), K 2 CO 3 (2.4eq), DMF, reacted for 1 hour, washed to remove DMF, and separated by column chromatography (silica gel: 200~300 mesh, eluent volume ratio: petroleum ether: acetone = 3:1), to obtain a light yellow oily liquid The pure product is dimethyl 4-oxo-2-((4-phenoxyphenyl)amino)pimelate. The yield was 81%.
[0032] The NMR data of this compound are as follows: 1H NMR (600 MHz, CDCl3) δ 7.28 – 7.25 (m, 2H),7.00 (t, J = 7.4 Hz, 1H), 6.91 (dd, J = 8.6, 0.9 Hz, 2H), 6.89 (d, J = 8.8Hz, 2H), 6.65 (d, J = 8.9 Hz, 2H), 4.42 (d, J = 6.7 Hz, 1H), 4.32 (s, 1H), 3.74 (s, 3H), 3.66 ...
Embodiment example 3
[0033] Example 3: Synthesis of dimethyl 2-((4-iodophenyl)amino)-4-oxopimelate
[0034]
[0035] The synthetic route and separation method are the same as in Example 2, wherein only the raw material 2-((4-phenoxyphenyl)amino)methyl acetate is replaced with 2-(4-iodophenylamino)methyl acetate. The pure product, dimethyl 2-((4-iodophenyl)amino)-4-oxopimelate, was obtained as light yellow oily liquid. The yield was 80%.
[0036] The NMR data of this compound are as follows: 1 H NMR (600 MHz, CDCl 3 ) δ 7.43 – 7.40 (m, 2H),6.42 (d, J = 8.7 Hz, 2H), 4.46 (d, J = 8.7 Hz, 1H), 4.41 – 4.37 (m, 1H), 3.72(s, 3H) , 3.65 (s, 3H), 3.01 (dd, J = 5.0, 3.1 Hz, 2H), 2.74 – 2.70 (m, 2H),2.59 (t, J = 6.5 Hz, 2H). 13 C NMR (151 MHz, CDCl 3 ) δ 206.1, 172.9, 172.8, 146.0, 137.9, 115.8, 79.5, 52.6, 52.4, 51.7, 44.5, 37.6, 27.6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com