Kit for detecting human parvovirus IgM antibody
A parvovirus and kit technology, which is applied in the field of kits for detecting human parvovirus IgM antibodies, can solve the problems of narrow linear range, inaccurate sample addition, and human errors in naked eye interpretation results, and achieve wide detection linear range and high detection results. Accurate, short detection time effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1 Preparation of a kit for detecting human parvovirus IgM antibody
[0017] 1. Preparation of B19-IgM magnetic particle suspension
[0018] 1.1 Take 30ul of the mixed carboxy surface magnetic bead stock solution and wash 5 times with 300ul of PBS buffer.
[0019] 1.2 Activate the magnetic particles with 20mg / ml EDC and 20mg / ml NHS for 1 hour.
[0020] 1.3 Wash the activated magnetic particles 3 times with pH 4.75 buffer B.
[0021] 1.4 Add mouse anti-human IgM μ chain monoclonal antibody according to the amount of 0.25ug / test, and coat for 2 hours.
[0022] 1.5 Seal 4 times with sealing solution.
[0023] 1.6 Add 3ml of sealing solution for preservation.
[0024] The sealing solution used is prepared by 0.01M PH7-8 PBS buffer solution, which contains 1‰ (v / v) preservative Proclin 300, 0.2‰ (w / v) preservative NaN 3 , 3% (w / v) stabilizer bovine serum albumin, 1‰ (v / v) surfactant Triton X-100, 5% (v / v) glycerin.
[0025] 2. Preparation of magnetic particle B1...
Embodiment 2
[0038] Embodiment 2 The usage method of kit of the present invention
[0039] 1. Sample requirements
[0040]1.1 Use correct medical technology to collect whole blood samples, centrifuge after the blood cells are coagulated at room temperature, and extract serum for testing. Plasma with EDTA (1.5g / L whole blood), sodium citrate (10.9mmol / L whole blood) or heparin sodium (0.1-0.2mg / mL whole blood) as anticoagulant can also be used for detection.
[0041] 1.2 Serum should not be left at room temperature for more than 8 hours after collection. If it is not tested within 8 hours, the sample should be placed in a refrigerator at 2-8°C; if it needs to be stored or transported for a long time, it should be frozen at below -20°C to avoid repeated freezing and thawing. Return to room temperature before use, shake gently to mix.
[0042] 2. Inspection method
[0043] 2.1 Consumables inspection: load or check whether the consumables are sufficient according to the instrument instruct...
Embodiment 3
[0053] Embodiment 3 Performance evaluation of the kit prepared by the present invention
[0054] (1) Analytical sensitivity
[0055] Use the 0-value calibrator as a sensitivity control product to measure 20 wells, calculate the mean (M) and standard deviation (SD) of the luminescence value, and calculate the concentration value of M+2SD according to the dose-response curve, and the result is for the analytical sensitivity.
[0056]
[0057] (2) Linear detection:
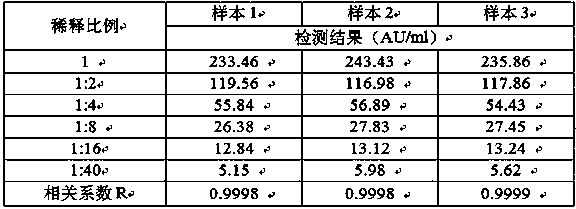
[0058] Dilute 3 high-value samples close to 240AU / ml into 6 concentrations according to 1:2, 1:4, 1:8, 1:16, 1:40, and samples with low-value concentrations must be close to the lower limit of the linear range ( 6AU / ml). The samples of each concentration were detected twice, and the average value was calculated, and the average value of the results and the dilution ratio were fitted with a straight line by the least square method, and the linear correlation coefficient R was calculated, and R≧0.99 was required....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com