Construction method and application of nano drug loading system targeting cell endoplasmic reticulum
A nano-drug-loading and endoplasmic reticulum technology, applied in the field of medicine, can solve problems such as insufficient attention, and achieve the effects of reducing toxic side effects, enhancing therapeutic efficacy, and increasing concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
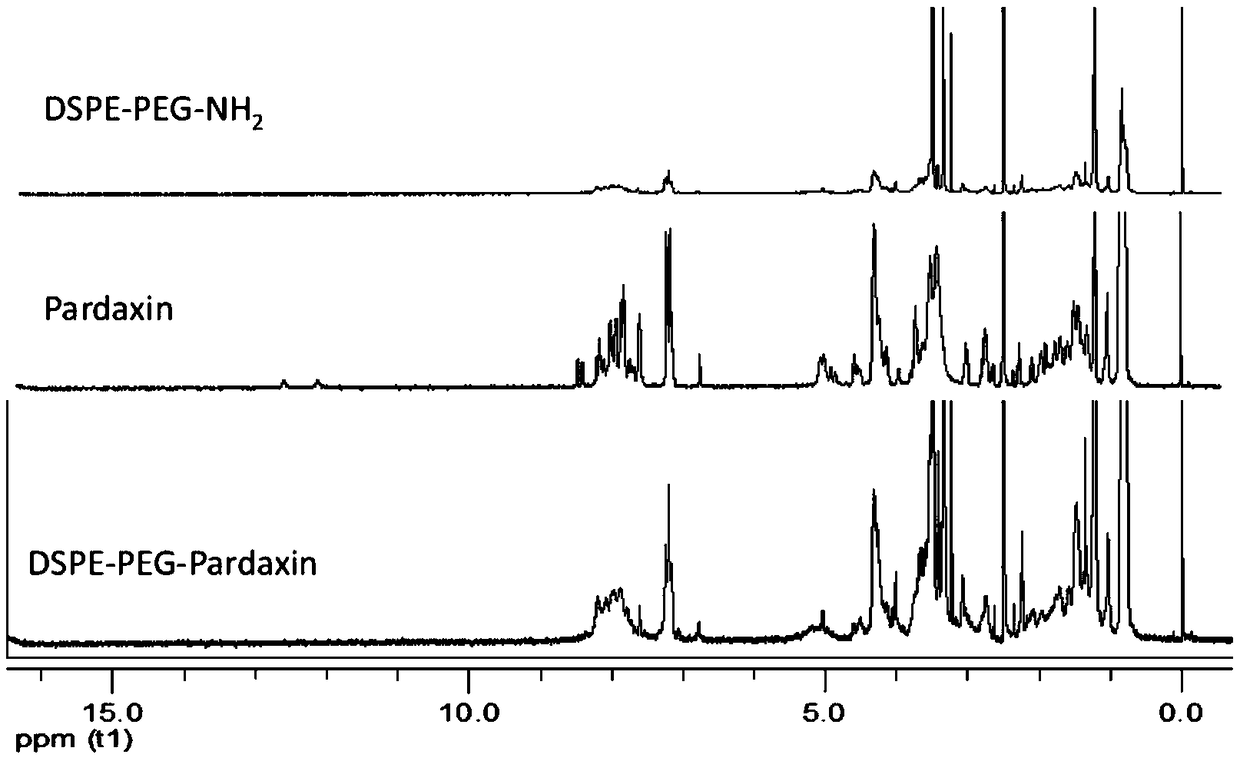
[0026] Synthesis of DSPE-PEG-Pardaxin: Precisely weigh Pardaxin, dissolve in 3mL-10mL anhydrous DMF solvent, add (BOC) 2 O reagent protects 4 free amino groups on Pardaxin polypeptide, (BOC) 2 The molar ratio of O:Pardaxin was 5.2:1, and the reaction was carried out under a nitrogen seal in the dark for 12 hours. by (BOC) 2 After protection by O reagent, EDC and NHS were added to activate the carboxyl group on the Pardaxin polypeptide, the molar ratio of EDC:Pardaxin was 10:1, and the molar ratio of NHS:Pardaxin was 5:1, and the reaction was activated at room temperature for 2 hours. After activation, add DSPE-PEG-NH 2 , reacted for 24 hours under magnetic stirring, DSPE-PEG-NH 2 :Pardaxin molar ratio is 1:1. After the reaction is over, stir the reaction with 1ml 12M HCI for 2 hours to remove the BOC protection, then use 3M NaOH (1.2g dissolved in 10mL water) to return to neutrality, dialyze, and freeze-dry to obtain DSPE-PEG-Pardaxin, the structural formula is as follows ...
Embodiment 2
[0031] Synthesis of Thioctic Acid(TA)-PEG-Pardaxin: Via NH 2 -PEG-NH 2 and Thioctic Acid (ThiocticAcid, TA) dehydration reaction to synthesize NH2-PEG-TA. First, TA, DCC, NHS (molar ratio: 1:5:10) were dissolved in DMF and stirred at 60°C for 2 hours to activate the carboxyl groups on TA. Then, add a certain amount of NH 2 -PEG-NH 2 (NH 2 -PEG-NH 2 :TA=1:2, mol / mol) and continued stirring for 24 hours. The crude product was dialyzed against distilled water for 48 hours and then lyophilized to give NH 2 -PEG-TA.
[0032] Before the synthesis of Pardaxin-PEG-TA, using the method described above, the amino group on the pardaxin peptide was also (BOC) 2 O protection. Afterwards, EDC and NHS (Pardaxin:EDC:NHS=1:5:10, mol / mol) were used to activate the carboxyl group on FAL. Then, add NH 2 -PEG-TA (Pardaxin:NH 2 -PEG-TA=1:1, mol / mol) and continued stirring for 24 hours. At the end of the reaction, the protecting group was removed using HCl and the pH was adjusted by Na...
Embodiment 3
[0034] Synthesis of PCL-PEG-Pardaxin: added in dark and ice bath (BOC) 2 O reagent protects the free amino group on Pardaxin, (BOC) 2 The molar ratio of O:Pardaxin was 5.2:1, and the reaction was carried out under a nitrogen seal in the dark for 12 hours. by (BOC) 2 After protection by O reagent, EDC and NHS were added to activate the carboxyl group on the Pardaxin polypeptide, the molar ratio of EDC:Pardaxin was 10:1, and the molar ratio of NHS:Pardaxin was 5:1, and the reaction was activated at room temperature for 2 hours. After activation, add polyethylene glycol-polycaprolactone (PCL-PEG-NH 2 ), reacted for 24 hours under magnetic stirring, PCL-PEG-NH 2 :Pardaxin molar ratio is 1:1. After the reaction, stir the reaction with 1ml 12M HCI for 2 hours to remove the BOC protection, then use 3M NaOH (1.2g dissolved in 10mL water) to neutralize, dialyze, and freeze-dry to obtain PCL-PEG-Pardaxin.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com