A kind of synthetic method of novel monoamine oxidase inhibitor morabetamide
A technology of monoamine oxidase and morapetamide, which is applied in the field of preparation of monoamine oxidase inhibitors, can solve the problems of cumbersome operation and low yield, and achieve the effect of simple reaction circuit, high yield and simple reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 Synthesis of 5-chloro-2-pyridinecarbonyl chloride (b)
[0021] Under the condition of 20-25℃, first add 5-chloro-2-pyridinecarboxylic acid into the solvent dichloromethane, add N,N-dimethylformamide (DMF) as a catalyst, stir evenly with magnetic force, and then add dropwise Thionyl chloride, no 吡啶羧酸 :n 二氯亚砜 =1:2 , the temperature of the reaction system is controlled at no more than 30°C, and the time for the dropwise addition of thionyl chloride is controlled at 10-15min. After the dropwise addition, the temperature was raised to reflux for 6-8 hours, and the reflux temperature was 40-45°C. After the reflux was completed, the solvent dichloromethane and excess thionyl chloride were removed under normal pressure, cooled, and vacuum-dried to obtain white crystals 5- Chloro-2-pyridinecarbonyl chloride. The yield is 98%, the purity is 99.4%, and the melting point is 218-220°C.
Embodiment 2
[0022] Example 2 Synthesis of 5-chloro-N-(2-hydroxyethyl)-2-pyridinecarboxamide (c)
[0023] First, 5-chloro-2-pyridinecarbonyl chloride (b) was added to the solvent dichloromethane, magnetically stirred to dissolve, and then ethanolamine was added dropwise, no 吡啶甲酰氯 :n 乙醇胺 =1:2 , the dropping time is controlled at 10-15 minutes, the reaction temperature is controlled at 40-50°C, and the reaction is continued at 40-50°C for 2h-4h after the dropwise addition is completed. After the reaction was completed, the solvent was removed under normal pressure, washed with water, filtered, dried, and recrystallized from toluene to obtain white crystal 5-chloro-N-(2-hydroxyethyl)-2-pyridinecarboxamide (c). The yield is 93%, the purity is 99.3%, and the melting point is 117-120°C.
Embodiment 3
[0024] The synthesis of embodiment 3 morapetamide (d)
[0025] Add 5-chloro-N-(2-hydroxyethyl)-2-pyridinecarboxamide (c) into the solvent toluene, first raise the temperature to let it dissolve, and then add dropwise morpholine and 98% concentrated sulfuric acid after completely dissolving, no 吡啶甲酰胺 :n 吗啉 =1:2 , heating and reflux reaction for 12-16h, the reflux temperature is 120-125°C. After the reaction is completed, cool, filter with suction, and recrystallize from toluene to obtain morapetamide as a white solid. The yield is 56%, the purity is 99.5%, and the melting point is 162-164°C.
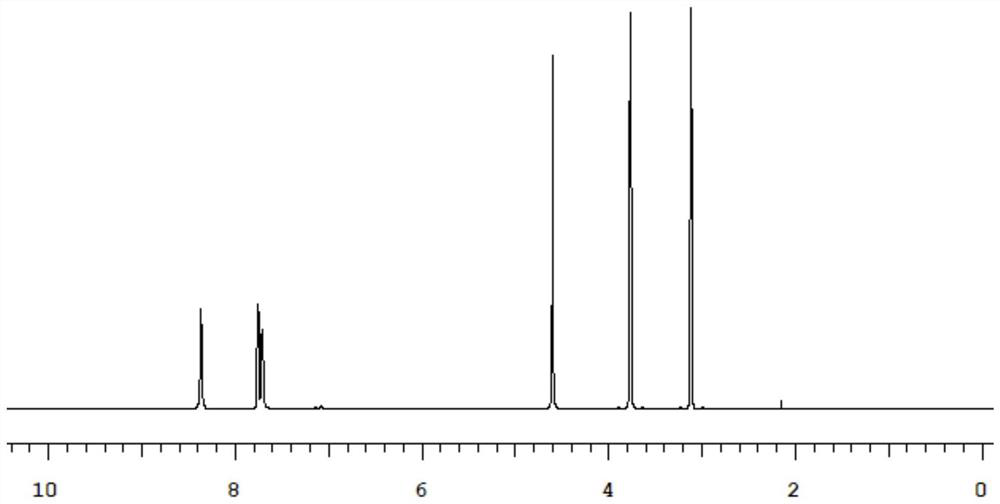
[0026] Its relative molecular mass detected by mass spectrometry is 268.72, its melting point is 162°C-164°C, and it is a white crystal; figure 1 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com