4-Chromanone compound containing trifluoromethyl and preparation method of 4-Chromanone compound
A technology for chroman and ketone compounds, which is applied in the field of chroman-4-one compounds and their preparation, and achieves the effects of mild reaction conditions and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] The described a trifluoromethyl-containing chroman-4-one compound and its preparation method comprise the following steps:
[0022] Disperse the aldehyde compound of structure (I), the trifluoromethyl reagent of structure (II) and the oxidizing agent in a solvent, and heat and stir to obtain trifluoromethylated chroman with structure (III) -4-Kone compounds:
[0023]
[0024] The specific structure of III is:
[0025]
[0026] Described oxidizing agent is potassium persulfate, sodium persulfate or ammonium persulfate;
[0027] The solvent is a mixture of acetonitrile, ethanol, isopropanol, N,N-dimethylformamide, acetone, 1,4-dioxane, dimethyl sulfoxide, ethyl acetate or dichloromethane and water ;
[0028] The molar ratio of the aldehyde compound to the trifluoromethyl reagent is 1:1-1:1.6;
[0029] The molar ratio of the aldehyde compound to the oxidizing agent is 1:1-1:3;
[0030] Described reaction temperature is 50 ℃-100 ℃;
[0031] The reaction time is ...
Embodiment 1
[0033] In a clean and dry 20mL Schlenk pressure-resistant reaction tube, add 1mmol allyl salicylaldehyde, 1.5mmol sodium trifluoromethanesulfinate, and 3mmol potassium persulfate successively, then add 1.5mL acetonitrile and 2.5mL water as solvents, The reaction tube was sealed and placed in an oil bath at 70° C. to react for 8 hours. After the reaction was finished, ethyl acetate was added to extract the reaction mixture, the organic phase was spin-dried by a rotary evaporator, and the residue obtained was separated by a silica gel column with petroleum ether and ethyl acetate as eluents, and the target product obtained was Colorless oily liquid, yield 62%.
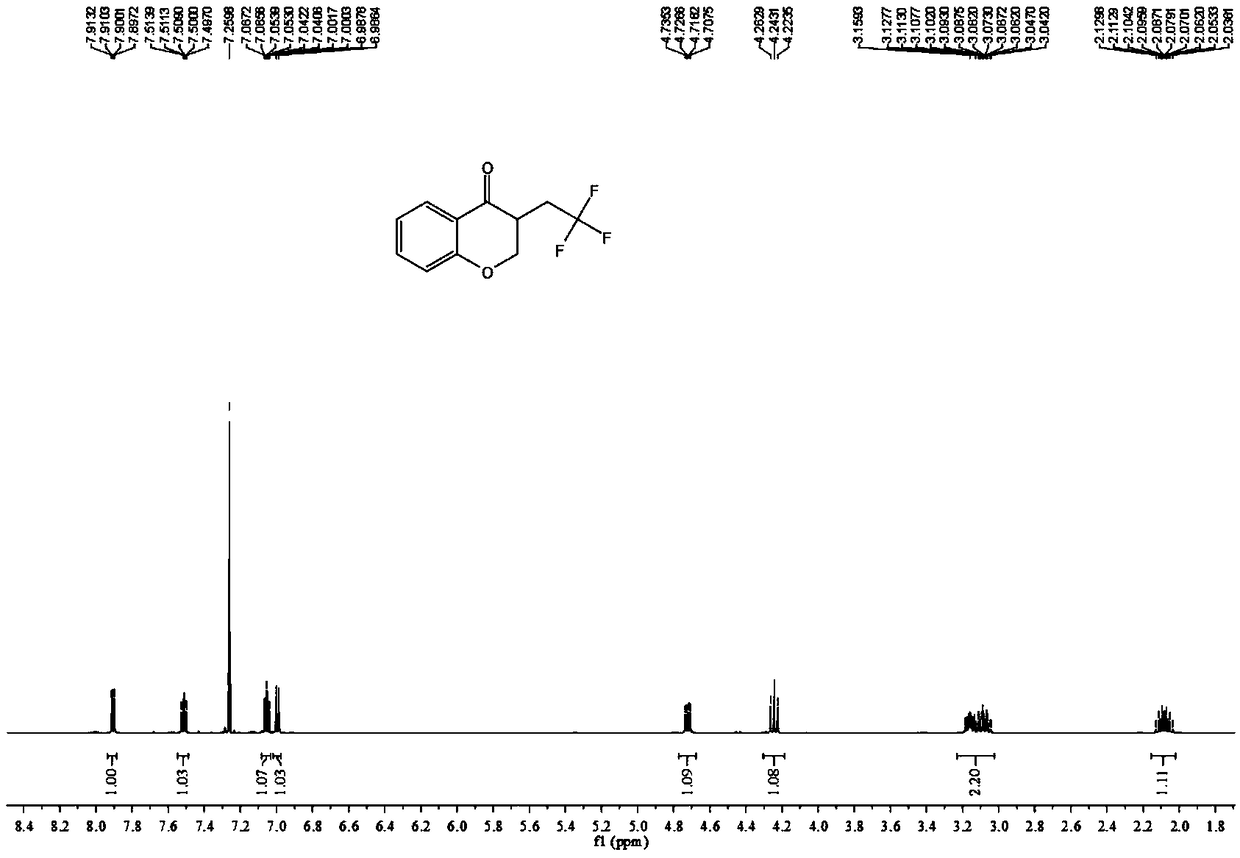
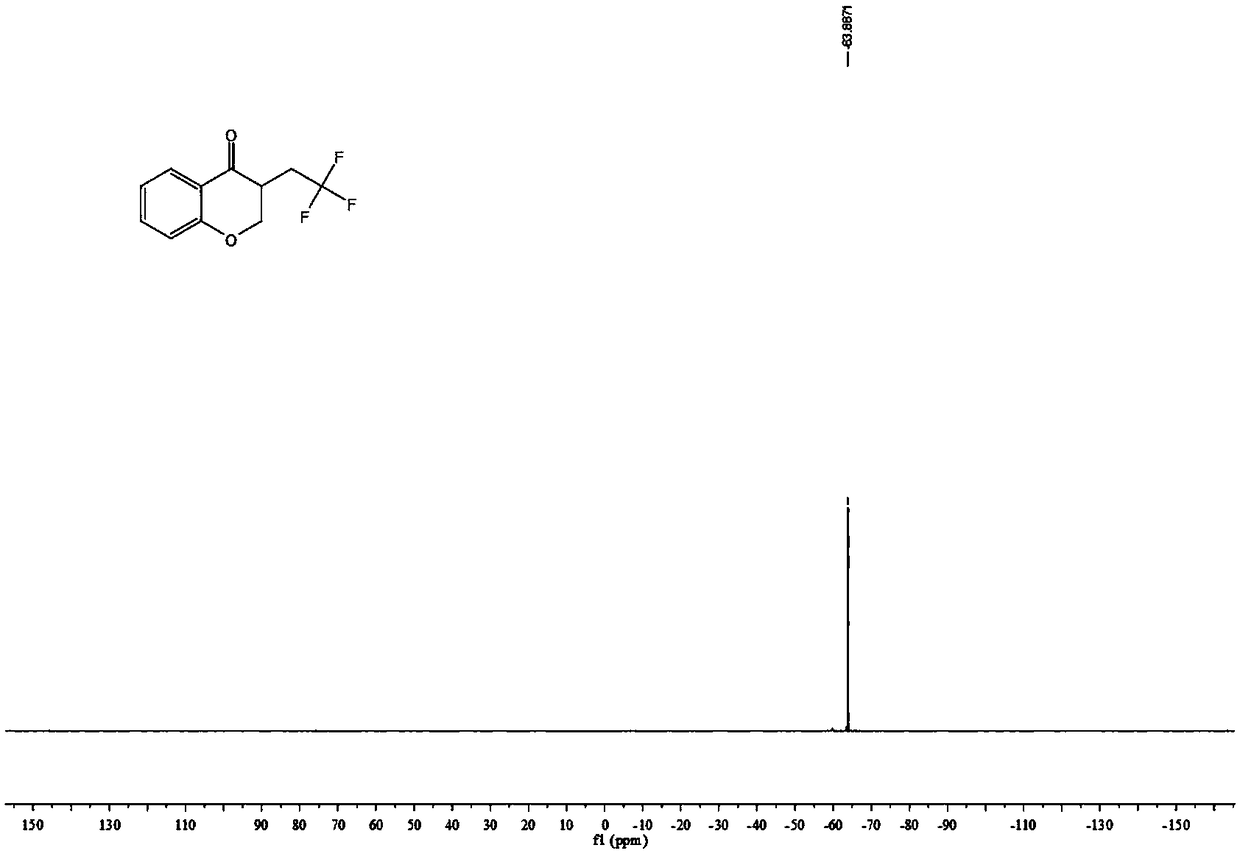
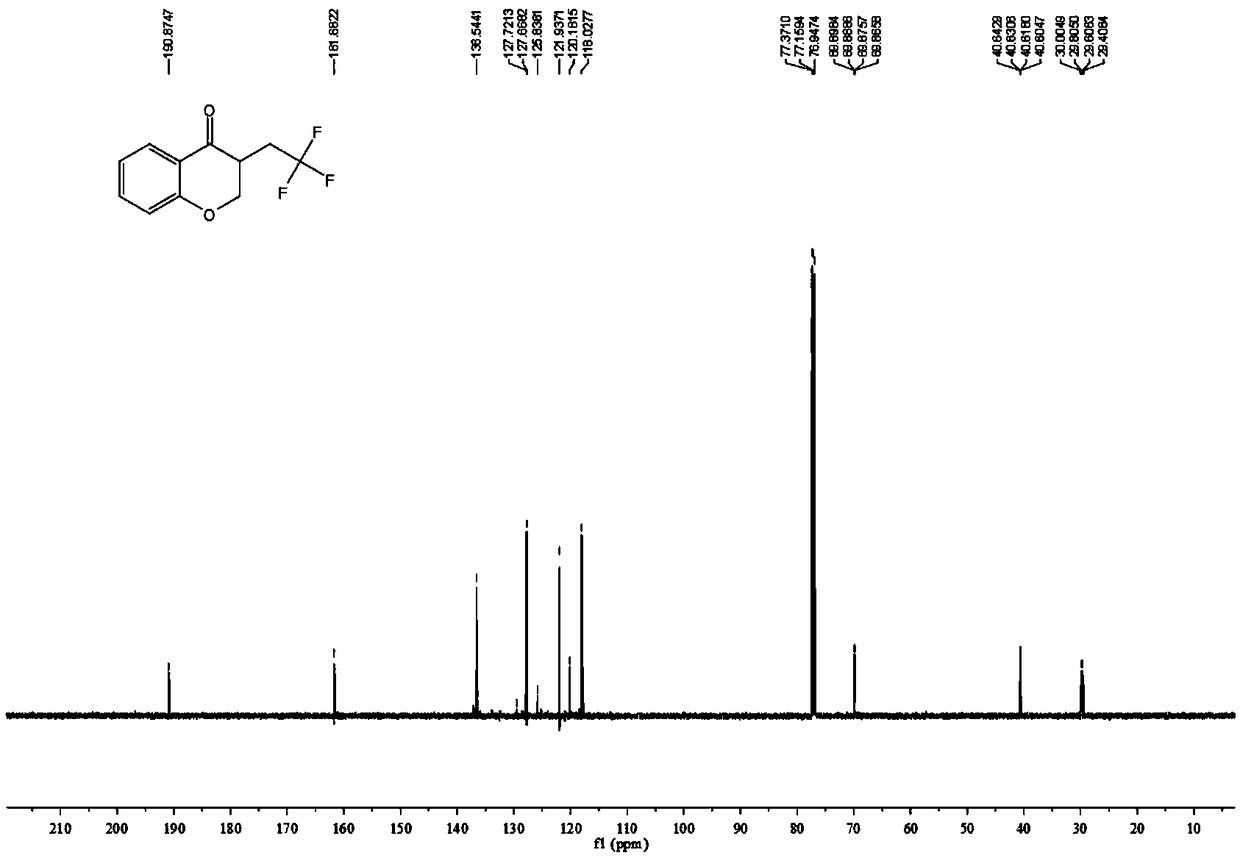
[0034] The proton nuclear magnetic resonance spectrum of the product prepared in this embodiment is as follows: Figure 1a As shown, the NMR fluorine spectrum is shown as Figure 1b As shown, the carbon NMR spectrum is as Figure 1c shown. It can be confirmed from the spectrum that the obtained product is the target c...
Embodiment 2
[0036] Add 1mmol 4-methoxyallyl salicylaldehyde, 1.5mmol sodium trifluoromethanesulfinate, 2.5mmol potassium persulfate, 1.5mL acetonitrile and 2.5 mL of water was used as a solvent, and the reaction tube was sealed and placed in an oil bath at 80° C. for 8 hours to react. After the reaction was finished, ethyl acetate was added to extract the reaction mixture, the organic phase was spin-dried by a rotary evaporator, and the residue obtained was separated by a silica gel column with petroleum ether and ethyl acetate as eluents, and the target product obtained was White solid, 38% yield.
[0037] The proton nuclear magnetic resonance spectrum of the product prepared in this embodiment is as follows: Figure 2a As shown, the NMR fluorine spectrum is shown as Figure 2b As shown, the carbon NMR spectrum is as Figure 2c shown. It can be confirmed from the spectrum that the obtained product is the target compound 2b.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com