Use of magnesium resources in serpentine to co 2 Methods for Mineralized Storage
A technology of serpentine and resource pairing, applied in the direction of magnesium carbonate, etc., can solve the problem of no leaching of serpentine and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
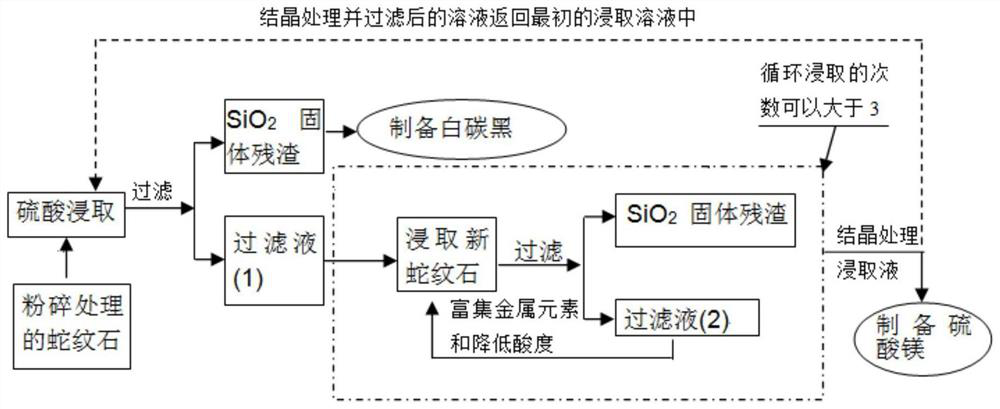
[0039] In this example, see figure 1 and figure 2 , a method of using magnesium resources in serpentine to CO 2 The method for carrying out mineralized storage comprises the following steps:
[0040] a. After the serpentine is crushed, ball milling is carried out, and the size of the particles is all passed through a 200-mesh sieve to obtain serpentine powder, and then the serpentine powder is calcined. The calcined treatment temperature is 500 ° C, and the calcined time is 180min, obtain calcined pre-activated serpentine powder; carry out cross-flow leaching of pretreated serpentine and sulfuric acid, the leaching conditions of pretreated serpentine and sulfuric acid:
[0041] The serpentine powder was added to the sulfuric acid solution for leaching reaction, the temperature was 50°C, the time was 250min, the solid-liquid mass-to-volume ratio of the pretreated serpentine and sulfuric acid was 0.1:10 (g / mL). The stirring speed of the mixed solution of serpentine and sulfu...
Embodiment 2
[0056] This embodiment is basically the same as Embodiment 1, especially in that:
[0057] In this embodiment, a kind of utilization of magnesium resources in serpentine to CO 2 The method for carrying out mineralized storage comprises the following steps:
[0058] a. After the serpentine is crushed, ball milling is carried out, and the size of the particles is all passed through a 100-mesh sieve to obtain serpentine powder, and then the serpentine powder is calcined. The calcined treatment temperature is 700 ° C, and the calcined time is 15min, obtain the serpentine powder of calcining pre-activation treatment; The serpentine after pretreatment and sulfuric acid are carried out cross-flow leaching, the leaching condition of pretreatment serpentine and sulfuric acid:
[0059] Serpentine powder was added to sulfuric acid solution for leaching reaction at a temperature of 100°C for 10 minutes. The stirring speed of the mixed solution of serpentine and sulfuric acid after treat...
Embodiment 3
[0063] This embodiment is basically the same as the previous embodiment, and the special features are:
[0064] In this embodiment, a kind of utilization of magnesium resources in serpentine to CO 2 The method for carrying out mineralized storage comprises the following steps:
[0065] a. This step is the same as in Embodiment 1;
[0066] b. dissolving the magnesium sulfate heptahydrate crystals obtained in the step a in water to obtain a magnesium sulfate solution, then adding the pretreated serpentine ore powder prepared in the step a to the magnesium sulfate solution, and inject CO into the magnesium sulfate solution 2 gas, controlling CO above the surface of the magnesium sulfate solution 2 The equilibrium pressure is 100Mpa, the temperature of the magnesium sulfate solution is controlled at 80°C, and the pH value is controlled in the range of 8-10 to ensure that Mg(HCO 3 ) 2 Formation, ball milling the mixed liquid system of magnesium sulfate and serpentine to accele...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com