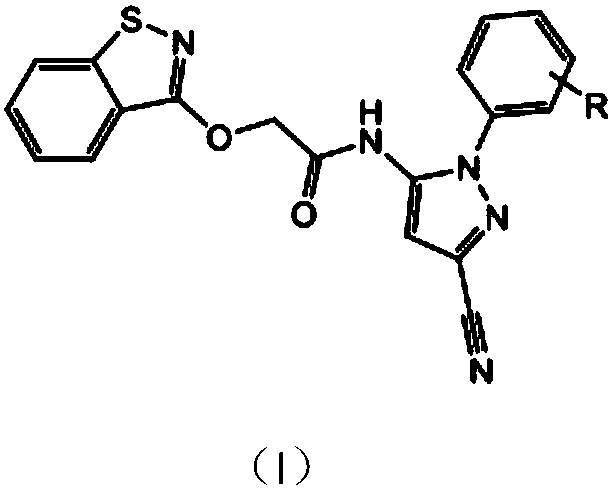
Compound having isothiazolinone and N-arylpyrazole structure and preparation method and application thereof
A technology for isothiazolinone and benzisothiazolinone sodium salt, which is applied to compounds with isothiazolinone and N-arylpyrazole structures and their preparation and application fields, and achieves simple and convenient reaction operation and reaction yield. High and good biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
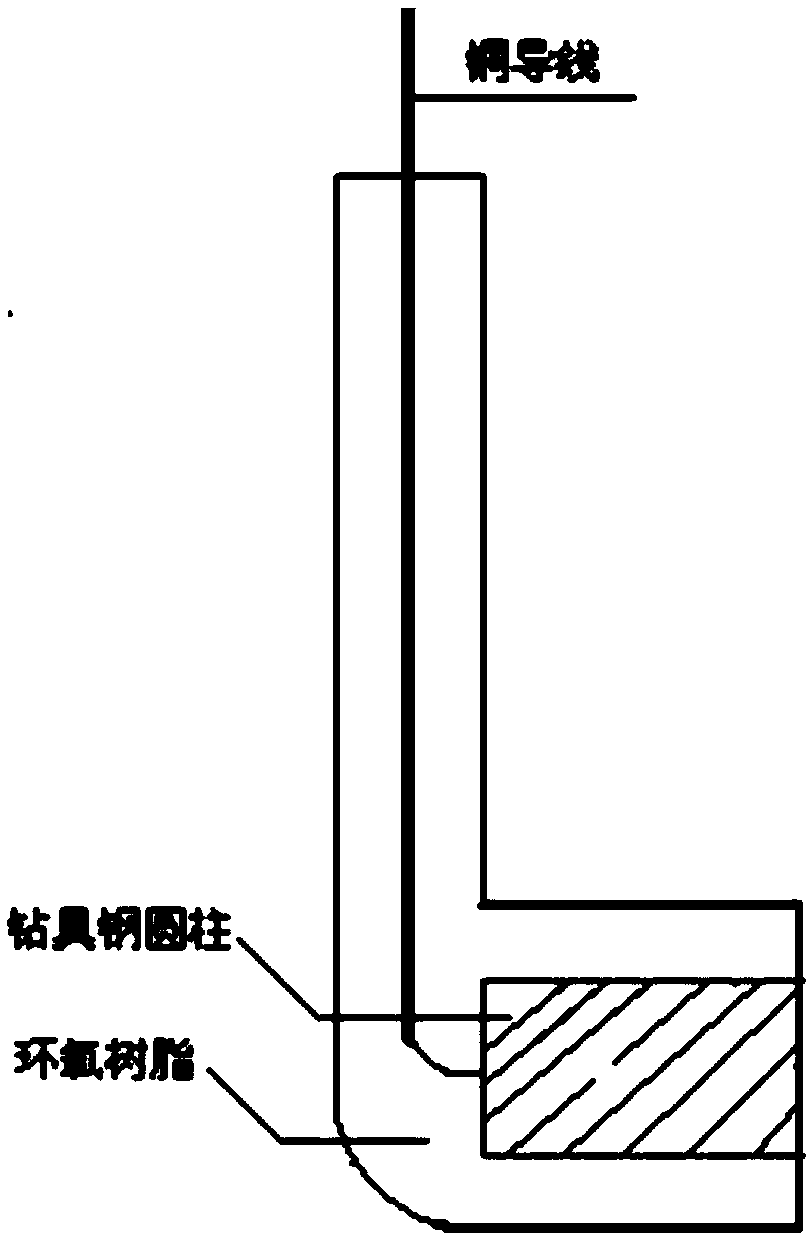
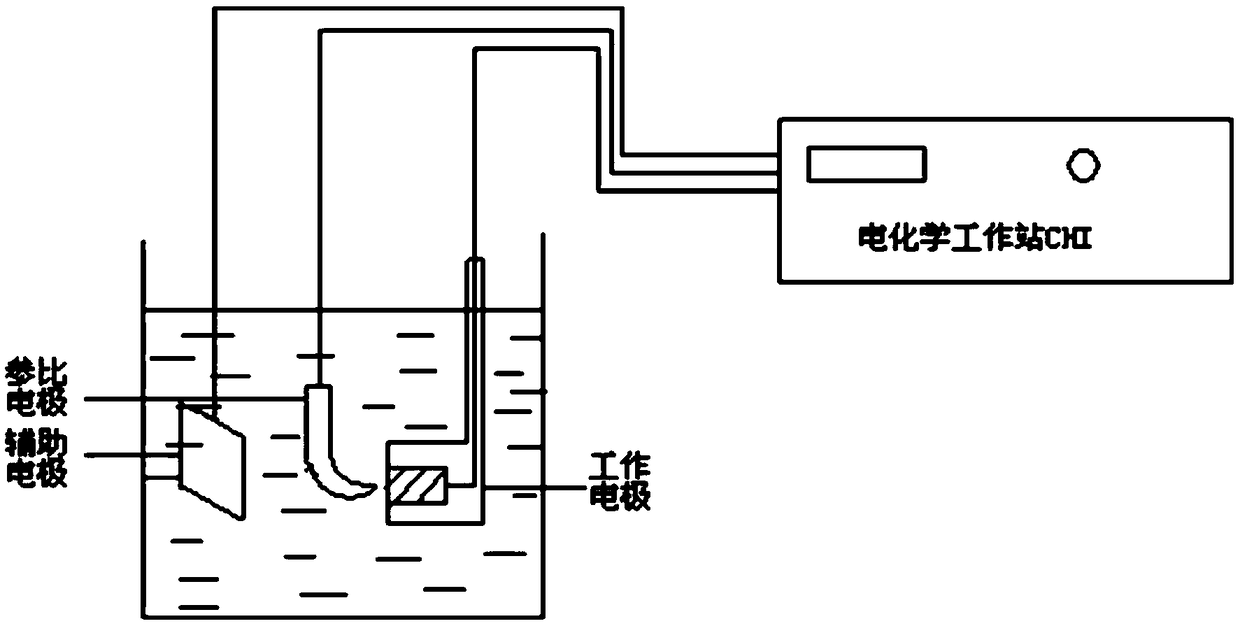
Image
Examples
Embodiment 1
[0028] 2-(Benzisothiazoline-3-oxyl)-N-(3-cyano-1-phenylpyrazol-5-yl)acetamide
[0029] In a 100mL four-neck flask, 3-cyano-5-amino-1-phenylpyrazole chloroacetamide and benzisothiazolinone sodium salt were dissolved in the organic solvent DMF according to the molar ratio of 1:1.1, and a small amount of potassium iodide was added As a catalyst, react in an oil bath at 100°C for 5h. After the reaction, the solvent was concentrated, poured into water, a large amount of solids precipitated, suction filtered, washed, and dried to obtain a crude product. Column chromatography (ethyl acetate:petroleum ether=1:1.2) gave the target product 2-(benzoiso Thiazolin-3-oxy)-N-(3-cyano-1-phenylpyrazol-5-yl)acetamide. Yield: 72%. Melting point: 155-157°C. The structural formula and spectrum analysis of the product are as follows:
[0030]
[0031] 1 H NMR (CDCl 3 ,500MHz)δ:5.15(s,2H,CH 2 ),7.15(s,1H,C–H),7.08~7.88(m,9H,Ar–H), 8.60(s,1H,N–H);
[0032] IR(KBr) v: 3177(N–H), 3058(C–H), ...
Embodiment 2
[0038] 2-(Benzisothiazolin-3-oxyl)-N-(3-cyano-1-(2-methoxyphenyl)pyrazol-5-yl)acetamide:
[0039] In a 100mL four-necked flask, 3-cyano-5-amino-1-2-methoxyphenylpyrazole chloroacetamide and benzisothiazolinone sodium salt were dissolved in the organic solvent DMF at a molar ratio of 1:1.4 , add a small amount of potassium iodide as a catalyst, and react in an oil bath at 100°C for 5h. After the reaction, the solvent was concentrated and poured into water. A large amount of solids precipitated out. Suction filtration, washing, and drying gave the crude product. Column chromatography (ethyl acetate:petroleum ether=1:1.3) gave the target product. Yield: 70%. Melting point: 152-154°C. The structural formula and spectrum analysis of the product are as follows:
[0040]
[0041] 1 H NMR (CDCl 3 ,500MHz)δ:3.84(s,3H,CH 3O),5.17(s,2H,CH 2 ),7.14(s,1H,C–H),6.78~7.87 (m,8H,Ar–H),8.31(s,1H,N–H);
[0042] IR(KBr) v: 3295(N–H), 3170(C–H), 2241(C≡N), 1713(C=O), 1596(C=N), 1557(C=C...
Embodiment 3
[0048] 2-(Benzisothiazolin-3-oxyl)-N-(3-cyano-1-(4-methoxyphenyl)pyrazol-5-yl)acetamide:
[0049] In a 100mL four-neck flask, 3-cyano-5-amino-1-p-benzyloxypyrazole chloroacetamide and benzisothiazolinone sodium salt were dissolved in the organic solvent DMF at a molar ratio of 1:1.1, Add a small amount of potassium iodide as a catalyst, and react for 5 hours in an oil bath at 100°C. After the reaction, the solvent was concentrated and poured into water. A large amount of solids precipitated out. Suction filtration, washing, and drying gave the crude product. Column chromatography (ethyl acetate:petroleum ether=1:1.2) gave the target product. Yield: 68%. Melting point: 171-172°C. The structural formula and spectrum analysis of the product are as follows.
[0050]
[0051] 1 H NMR (CDCl 3 ,500MHz)δ:3.85(s,3H,CH 3 O),5.14(s,2H,CH 2 ),6.99(s,1H,C–H),7.01~7.82(m,8H,Ar–H),8.34(s,1H,N–H);
[0052] IR(KBr) v: 3383(N–H), 3167(C–H), 2241(C≡N), 1717(C=O), 1597(C=N), 1557(C=C),...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com