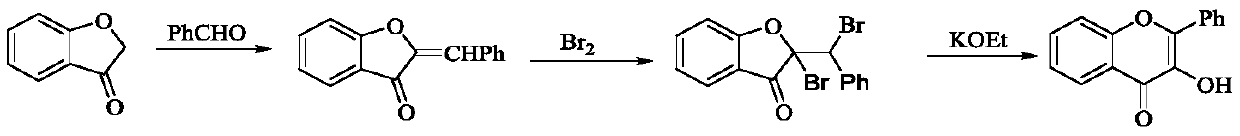
A kind of aqueous phase one-pot synthetic method of 3-hydroxyflavone and its derivatives
A technology of hydroxyflavone and synthesis method, which is applied in organic chemistry and other fields, can solve the problems of 3-hydroxyflavone's unknown adaptability, unknown substrate adaptability, unknown reaction mechanism, etc. High, simple and easy-to-control synthesis conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
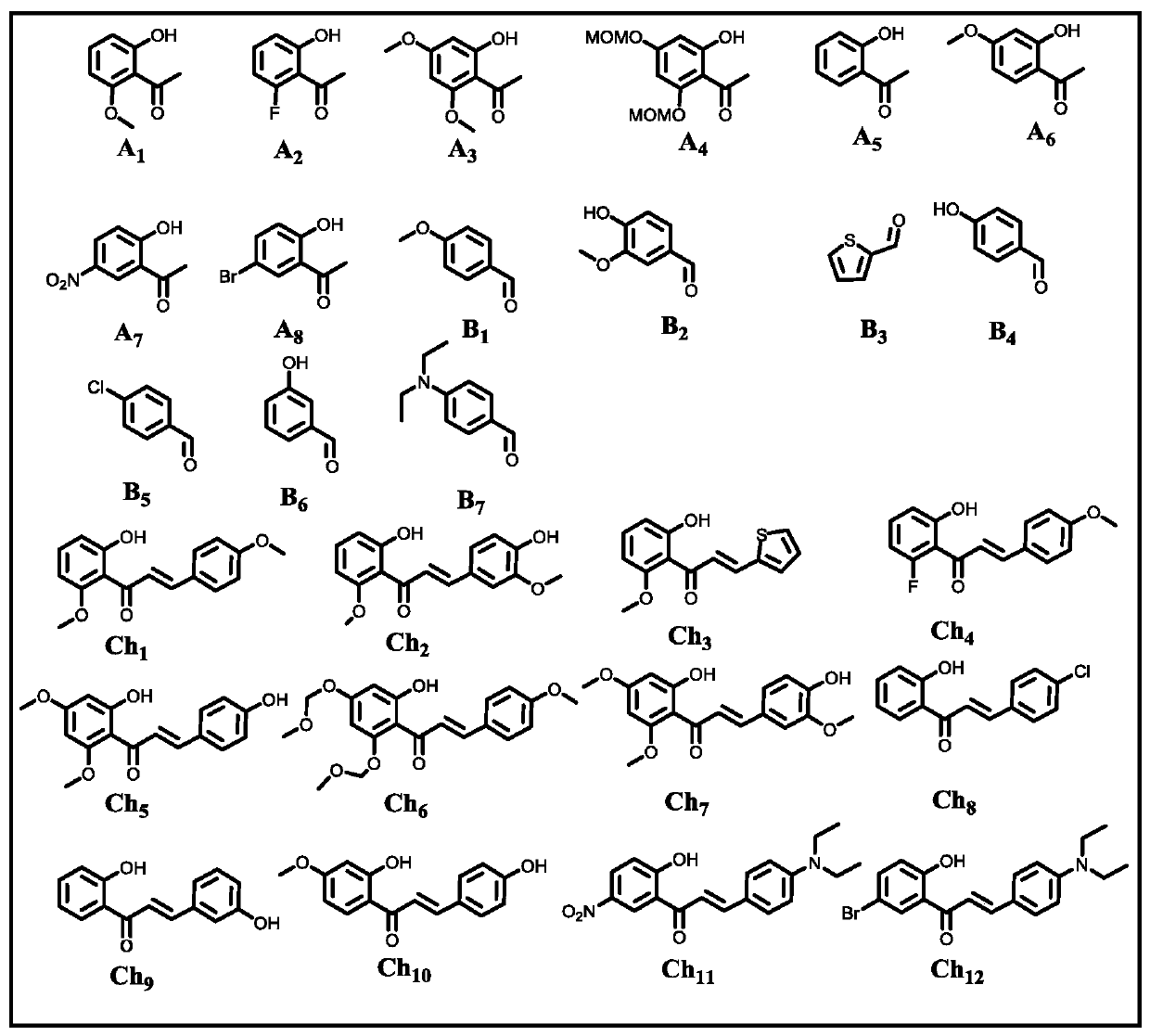
[0061] use figure 1 Raw material A in 1 and B 1 react. The reaction system includes: raw material A 1 (1.0mmol), B 1 (1.1mmol), base, reaction medium (10.0mL), and the reaction system was open to the atmosphere. Moore, A 1 :B 1 : Alkali=1:1.1:(2~16).
[0062] The reaction process is: after adding alkali into 10mL reaction medium, slowly add A 1 (1.0mmol), stir vigorously to dissolve and then slowly add B 1 (1.1mmol), then react at room temperature or by heating, the reaction system communicates with the atmosphere or oxygen, keep stirring for 24h, and the reaction ends.
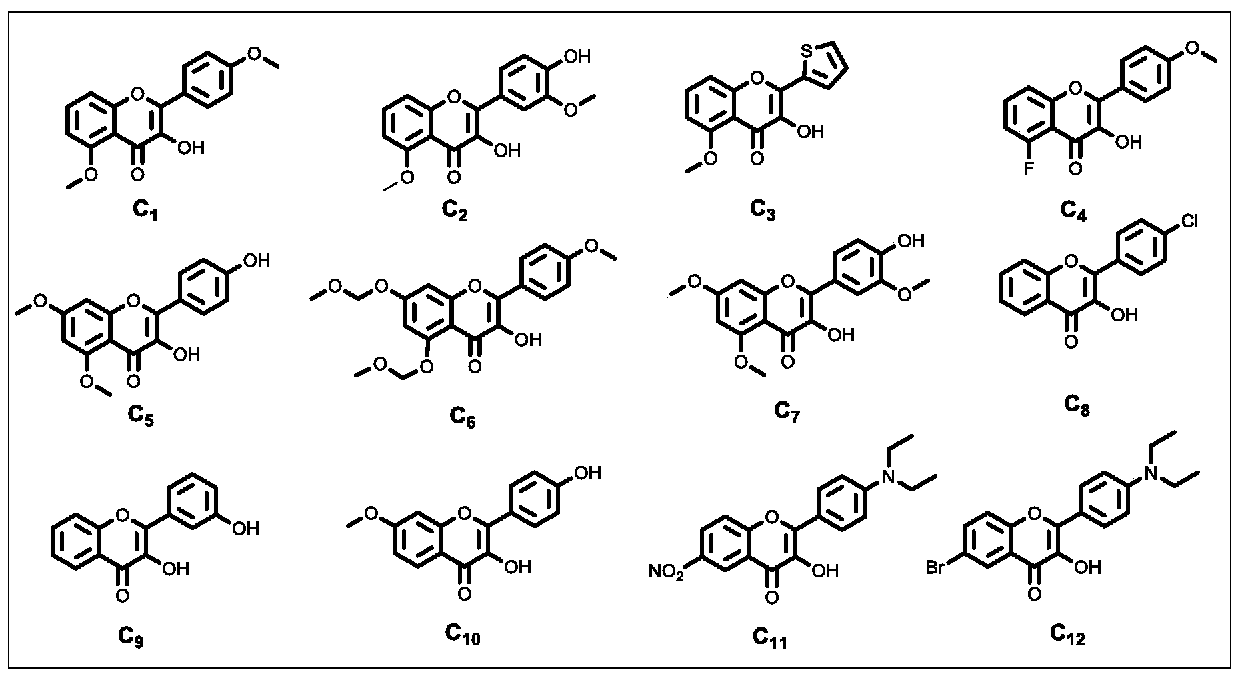
[0063] After the reaction, the reaction system was adjusted to pH≤7.0 with 6.0 mol / L hydrochloric acid aqueous solution, and the insoluble solid was filtered to obtain the crude product. The crude product was subjected to silica gel column chromatography (petroleum ether: ethyl acetate=4:1) to obtain the final product C 1 (Structural formula such as figure 2 shown). Wherein, the specific parame...
Embodiment 2
[0068] In addition to the different reaction raw materials, based on cost and environmental considerations, we chose the specific reaction conditions of Example Group 11 with lower energy consumption, less organic base consumption, and similar yields to carry out the reaction in this example. The structural formulas of raw materials A and B used in this embodiment are specifically as figure 1 Shown, the structural formula of each final product C produced is specifically as follows figure 2 shown. The specific reaction system is: raw material A (1.0 mmol), B (1.1 mmol), pyrrolidine (10.0 mmol), water (20.0 mL).
[0069] The concrete raw material A, B that each group of present embodiment is used, the product C of production and yield are as shown in table 2.
[0070] Table 2 Reaction system and yield comparison table
[0071] group raw material A raw material B final product C Yield (%) group 1 A 1
Embodiment 3
[0073] Except that the reaction raw materials are different, the same conditions as in Example Group 11 were selected to carry out the reaction of this example. The structural formula of each raw material Ch used in this embodiment is specifically as follows figure 1 Shown, the structural formula of each final product C produced is specifically as follows figure 2 shown. The specific reaction system is: raw material Ch (1.0 mmol), pyrrolidine (10.0 mmol), water (20.0 mL).
[0074] The specific raw materials Ch used in each group of the present embodiment, the product C produced and the yield are shown in Table 3.
[0075] Table 3 reaction system and yield comparison table
[0076] group Raw material Ch final product C Yield (%) group 1 Ch 1
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com