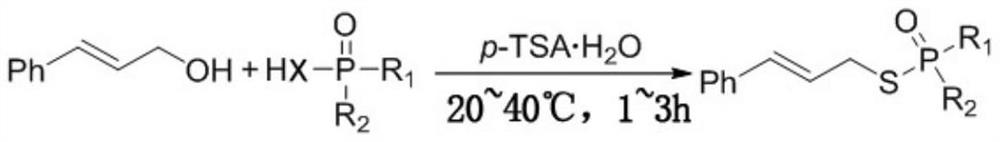
A kind of preparation method of allyl thio or selenophosphate and phosphonate
A technology of selenophosphorous ester and allyl sulfur, which is applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., can solve problems such as no one reported, and achieve substrate adaptability Strong, responsive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The preparation method of S-allyl diphenyl phosphonothioate comprises the following steps:
[0023] Weigh 0.33mmol diphenylthiophosphonic acid and 0.3mmol cinnamyl alcohol into a test tube, then add 0.3mmol p-TSA . h 2 O, seal the test tube, and carry out a stirring reaction at 30° C. for 1 h to obtain a reaction solution.
[0024] The obtained reaction solution was first dissolved in ethyl acetate, then washed with saturated brine, the organic phase was collected, dried and concentrated, and ethyl acetate / petroleum ether = 1 / 3 (v / v) was used as a developing solvent to analyze the concentrate Separation by column chromatography gave 81.9 mg of the target product.
[0025] The target product yield of this embodiment is 78%.
[0026] NMR characterization was carried out on the target product, the results are as follows: 1 H NMR (500MHz, CDCl 3 ):δ7.89(dd,J=12.9,7.3Hz,4H),7.49-7.47(m,2H),7.46-7.41(m,4H),7.25-7.22(m,2H),7.20-7.17(m ,3H),6.37(d,J=15.6Hz,1H),6.07(dt,J=1...
Embodiment 2
[0028] S-allyl O, the preparation method of O-diethyl phosphorothioate comprises the following steps:
[0029] Weigh 0.3mmol O, O-diethyl S-hydrothiophosphoric acid, 0.33mmol cinnamyl alcohol in a test tube, then add 0.3mmol p-TSA . h 2 O, seal the test tube, and carry out a stirring reaction at 30° C. for 1 h to obtain a reaction solution.
[0030] The obtained reaction solution was first dissolved in ethyl acetate, then washed with saturated brine, the organic phase was collected, dried and concentrated, and ethyl acetate / petroleum ether = 1 / 3 (v / v) was used as a developing solvent to analyze the concentrate Separation by column chromatography gave 69.6 mg of the target product.
[0031] The target product yield of this embodiment is 82%.
[0032] NMR characterization was carried out on the target product, the results are as follows: 1 H NMR (500MHz, CDCl3): δ7.34(d, J=7.5Hz, 2H), 7.29(t, J=7.5Hz, 2H), 7.22(t, J=7.2Hz, 1H), 6.57(d, J=15.7Hz, 1H), 6.25(dt, J=15.3, 7.5Hz,...
Embodiment 3
[0034] S-allyl O, the preparation method of O-diisopropyl phosphorothioate comprises the following steps:
[0035] Weigh 0.3mmol O, O-diisopropylthiophosphonic acid and 0.3mmol cinnamyl alcohol in a test tube, then add 0.33mmolp-TSA . h 2 O, seal the test tube, and carry out a stirring reaction at 30° C. for 1 h to obtain a reaction solution.
[0036] The obtained reaction solution was first dissolved in ethyl acetate, then washed with saturated brine, the organic phase was collected, dried and concentrated, and ethyl acetate / petroleum ether = 1 / 3 (v / v) was used as a developing solvent to analyze the concentrate Separation by column chromatography gave 82.8 mg of the target product.
[0037] The target product yield of this embodiment is 88%.
[0038] NMR characterization was carried out on the target product, the results are as follows: 1 H NMR (500MHz, CDCl 3 ): δ7.36(d, J=7.3Hz, 2H), 7.30(t, J=7.5Hz, 2H), 7.23(t, J=7.2Hz, 1H), 6.59(d, J=15.6Hz, 1H ), 6.27(dt, J=15.4, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com